Understanding Mycobacterial Structure
Mycobacteria cells are covered by mycolic acids, these mycolic acids are different from each other, M.alvei contains all kind of mycolic acids while M.tuberculosis contains alpha, methoxy and mycolic acids. ALVEI contains all kinds of acids. Mycobacterium alvei (M.alvei) belongs to the class of gram-positive aerobic bacteria known as the Mycobacteria, and Mycobacterium leprae, the causative agent of leprosy, are other recognized members of this genus. The two units of these mycolic acids are: the meromycolate and mycolic motif are the structure of these fatty acids which is responsible for the bacteria's resistance to antibiotics and other medicines.
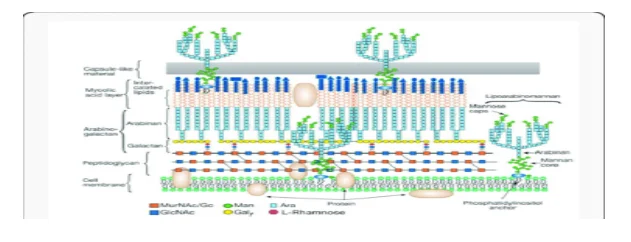
The Mycobacteria genus is a family of gram-shaped, aerobic, rod-formed (some of which are microaerophile)1. There are currently over 140 known species of Mycobacterium, 2, 3. This kind of bacterium may be devised into two categories: fast-growers (taking less than 7 days to make colonies and slow-growers (taking up two different types of Mycobacterium)4. The Mycobacteria cell shell is particularly important because its construction makes drug-irrenable bacteria5 this shell is of four layers: the inner plasma membrane, the cell wall composed of peptidoglycan and these are cellular-based saprophytes which are commonly dispersed in nature and have been shielded from various origins such as dirt, natural waters and mud as shown in the figure 1. They therefore contribute to the survival of the mycobacterium type 7 and therefore make diseasing conditions such as TB difficult to treat because of the rigidity of the cell wall of these mycolic acids and their fact that they are hydrophobic makes it difficult for antibiotics and other medicines to penetrate the cell wall 8.


The mycobacterium (M. alvei) is a non-tuberculosis and causes mycobacteriosis 13,14,15. The mero mycolate chain forms a 42-62 carbon chain with a number of functional groups while the mycolic motif complex includes an α-alkyl chain consisting of 22-26 carbon, and a β-hydroxy group. 6 The α-alkyl β-hydroxy is constantly discovered in the R, R configuration (see Figure-a-hydroxy), and the R configuration (figuo-a-a-alkyl β-hydroxy). The active version of the isoniazid prodrug that is used in the treatment of mycobactorial infections is identical to the triggered type of the EtaA. 10 Mycolic acids consist of two groups, mero Mycolate chain and Mycolic motif,11 and the Mycobacterial chain configurations vary in the Mycobacterium genus whi 9 The configurations of the myco-microphilate chain are distinct.
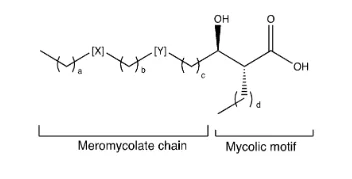
Since their chemical and biological properties may be studied, synthesizing single enantiomers of mycolic acids is important 16. This will contribute to new approaches for mycobacterial disease diagnosis and treatment, and allow for the identification between specific mycobacteria.
Take a deeper dive into Acute Porphyria: Symptoms, Causes, and Diagnosis with our additional resources.
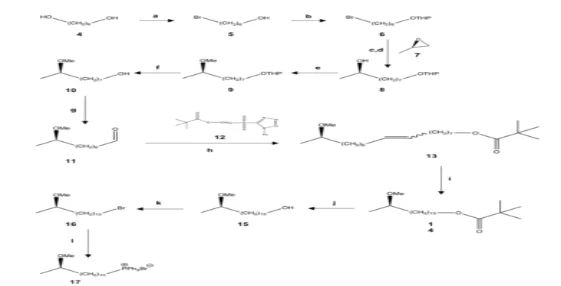
This investigation was aimed at production of sulfone 3, which constitutes the distal position of os-1 methoxy mycolic acid from M in the meromycolate chain. This has been achieved in three main parts: first the phosphonium salt synthesis 17; the second the aldehyde synthesis 19; and the third the phosphonium salt synthesis. The last element is the relation between 2 units (17 and 19) and the ester 20, then transformed in a 3-step synthesis into sulfone 3 (Schemes 1 and 2). When the required sulfone is synthesized, it is then used to make a full enantiopure mycological acid in the synthesis for the rest of the meromycolate chain that is then associated with the mycolic motif.
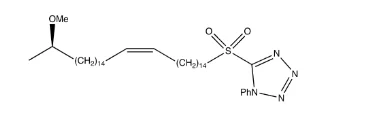
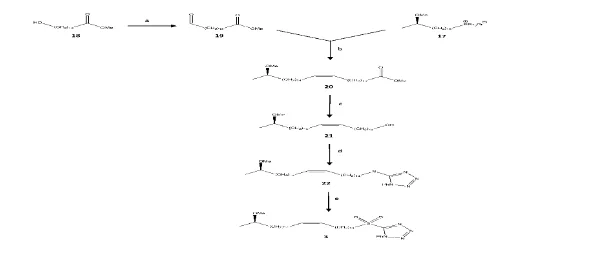
A coupled reaction between aldehyde 11 and C8 sulfone 12 by means of the modified Julia reaction alkene 13 developed with mixtures of cis / Trans isomers.18, 19, 20 It was shown that the modified Julia reaction was efficient, as 13CNMR shown a new peak of 1302 suggesting a double carbon-carbon bond. This couplage reaction was shown to be effective as there were signalisations from both compounds used in the experiment, such as sharp singles, at 3:20 ppm showing (R) methoxy and large pentets under it belonging to the experiment Phosphonian salt 17 and Aldehyde 19 were then mixed using sodium bis (trimethylsilyl) amide to create ester 20 in its configuration of cis.19,20 The reaction was effective as the group of signals at 9,77 ppm and 7,86 ppm belonging respectively to the aldehyde and triphenyl groups are no longer visible 21. Due to unforeseeable circumstances, the period was shortened in the laboratory, preventing sulfone 3 from being formed, which would result in an ester reduction into 21 and a substitution for 21 alcohols. Diol 4 was brominated in bromide 5 with HBr, brominated in a secondary primary alcohol with an acetal 6 conversion using 3,4-Dihydro-2H-pyrane which is important because it avoids methylation of the primary alcohol.17 Diol 4 was added in the first place during a (R)-propylene-7 ring opening by means of a Grignard reagent in order. The developed secondary alcohol was methylated into the (R) methoxy group of compound 9, accompanied by a de-protection from acetal to alcohol 10 which is subsequently oxidized using aldehyde 11 ready-to-connect pyridinium chlorochromate (PCC).
Experimental
General Procedure
Thin-layer (TLC) chromatography is conducted on silica gel plates from Merck-Millipore. The findings were presented with a potassium permanganate solution, or an industrial methylated spirit solution with phosphomolybic acid (Ims) heated at the time. All the chemical products used in this synthesis have been developed in high purity from the sodium wire in Sigma Aldrich, all solvents were derived from the sodium sample of Thermo Fischer, solvents that needed to be dried, such as tetrahydrofuran (DHF) were dried using a sodium wire with nitrogen.
6-bromohexan-1-ol (5)
The mixture was left to reflux during 18 hours with a microwave to add 1.6-Hexanediol (100 g, 0.846 mol) to toluene (300 mL). The toluene film was then decanted and the aqueous coating re-extracted with toluene (2 Tab-100 mL) until reflux is moved through a separating funnel. The combined organic layers have been extracted through distillation with NaHCO3 (150 mL). The base oil had been extracted by chromatography on columns and eluting petrol / ethyl acetate (10:1) for yielding 6-bromohexane-1-ol (66.09 g, 0,265 mol, 43 percent) colorless oil. This showed: μH (400 MHz, CDCl3): 3.65 (2H, t, J 6.5 Hz), 3.42 (2H, t, J 6.7 Hz), 1.91-1.84 (2H, m), 1.62-1.55 (2H, m), 1.50-1,37 (5H, m);
2-((6-bromohexyl)oxy)-tetrahydro-2H-pyran (6)
The mixed organic layers were washed, evaporated and flashed out and then treated with petrol / ethyl acetate, by means of column chromatography. (10:1) to yield a colourless oil 2-((6-bromohexyl)oxy)-tetrahydro-2H-pyran (65.29 g, 0.246 mol, 67%); which showed: δH (400 MHz, CDCl3): 4.55 (1H, m), 3.86-3.81 (1H, m), 3.74-3.69 (1H, dt, J 9.6, 6.7 Hz), 3.49 (1H, m), 3.39-3.36 (3H, t, J 6.8 Hz), 1.87-1.80 (3H, m), 1.72-1.66 (1H, m), 1.60-1.38 (10H, m); δc (101 MHz, CDCl3): 99.9, 67.4, 62.4, 33.9, 32.8, 30.8, 29.6, 28.1, 25.5, 25.4, 19.7; vmax/cm-1: 2916, 2848, 1469, 1022; [α]D24.3 + 0.32° (c 1.50 g/mL, MeOH).
(2R)-9-((tetrahydro-2H-pyran-2-yl)oxy)nonan-2-ol (8)
In the combined organic compounds, petrol / ethyl acetate was first washed, evaporated and processed by means of column chromatography to create colorless liquid, (2R)-9-(((tetrahydr 2-H-pyrene-2)oxy)-2-ol, which revealed that the impurities were first taken off and petrol / ethyl acetate (5:1) had been taken off (6.41 g, 0.0263 mol or 32%)δH (400 MHz, CDCl3): 4.58 (1H, dd, J 2.6 Hz), 3.87 (1H, m), 3.76-3.70 (1H, dt, J 9.5, 6.9 Hz), 3.53-3.48 (1H, m), 3.41-3.35 (1H, dt, J 9.6, 6.6 Hz), 1.85 (1H, qd, J 3.7 Hz), 1.75-1.69 (1H, m), 1.61-1.54 (8H, m), 1.40-1.31 (13H, m), 1.19 (3H, d, J 6.2 Hz); δC (101 MHz, CDCl3): 99.1, 68.3, 67.8, 62.5, 39.5, 30.9, 29.9, 29.7, 29.6, 26.3, 25.8, 25.6, 23.6, 19.9; vmax/cm-1: 3409, 2927, 2854, 1022; [α]D24.5 – 4.3° (c 1.89 g/mL, MeOH). The solvent was applied to the fluid dropwise retain a temperature at -30 ° C after the water has been drained, which is therefore to hit room temperature (R)-[+] propylene oxide (2.1 g and 0.0362 mol). Dissolved dry THF (10 mL) The magnesium suspension was then refluxed in a dry THF 2-(((6-bromohéxy)oxy)-tetrahydro-2, pyrene (21,75 g,0,0828 mol) drop-way, which was then refluxed for thirty minutes with a petroleum bath at 130 ° C. Dissolved into dry THF (200 mL) a Copper Iodide solution (1.72 g, 0.00905 mol) was cooled to -35 ° C and the Grignard reagent was applied dropwise and then agitated for 30 minutes.
2-(((R)-8-methoxynonyl)oxy)tetrahydro-2H-pyran (9)
Ethyl acetate was used to decipher the aqueous layer and re-extract the mixed organic compounds, dry up, evaporated, and filtered by column chromatography, first with petrol / ethyl acetate (10:1), accompanied then by petrol / ethyl acetate (5:1) to create a colorless oil: 2-(((((R)-8-methoxynonyl)oxy-tetrahydro-2H-pyrene (4.67 g, 0.0181 mol, 68 percent)) showed: δH (400 MHz, CDCl3): 4.58 (1H, dd, J 2.6 Hz), 3.89 (1H, m), 3.76-3.70 (1H, dt, J 6.9, 9.6 Hz), 3.53-3.47 (1H, m), 3.41-3.35 (1H, dt, J, 6.7, 9.5 Hz), 3.31 (3H, s), 3.29 (1H, br, p, J 6.2, 5.4 Hz), 1.85-1.80 (1H, m), 1.74-1.69 (1H, m), 1.64-1.52 (8H, m), 1.36-1.29 (10H, m), 1.12 (3H, d, J 6.1Hz); δc (100 MHz, CDCl3): 99.0, 77.0, 67.8, 62.5, 56.1, 36.5, 30.9, 29.9, 29.8, 29.6, 26.3, 25.6, 25.5, 19.8, 19.1; vmax/cm-1: 2928, 2855, 1022; [α]D24.8 – 2.4° (c 2.15 g/mL, MeOH).
(R)-8-methoxynonan-1-ol (10)
Column chromatography was applied for the combined organic layers and the first to remove petroleum-ethyl acetate (5:1) followed by petroglyph (5:2) to produce colorless petroleum (R)-8-methoxynon- 1-ol (2.80 g, 0.0161 mol, 89%) showing: β (400 MHz, CCL3): 3.60 (2H, t, J 6.6 Hz), 3.29 (3H, s), 3.26 (1H, br, p, J 6.1 Hz), 1.83 (1H, br, s), 1.53 (3H, m), 1.36-1.27 (10H, m), 1.10 (2H, d, J 6.1 Hz); δc (100 MHz, CDCl3): 76.8, 62.8, 55.8, 36.2, 32.6, 29.6, 29.3, 25.6, 25.3, 18.9; vmax/cm-1: 3306, 2929, 2856, 1633, 1529; [α]D25 – 3.2° (c 1.86 g/mL, MeOH).
(R)-8-methoxynonanal (11)
A 10:1 petrol solution: ethyl Acetate, then filtered by the silica-pad and then evaporated to produce colored oils (1.56 g, 0.0091mol, 57%) that showed: ̈H (400 MHz, CDCl3): 9.76(1H, t, J 1.8 Hz), 3.31(3H, s), 3.30-3.25(1H, br, p, J 5.2 Hz), 2,45- 2.40(2H, TD, J 7.3, 1.9 Hz), 1.63 (3H, m), 1.38-1,30 (10H, m), 1.12 (3H, p, J 5.2 Hz), 1.45-2.4
(R)-16-methoxyheptadec-8-en-1-yl pivalate (13)
Crude products were distilled with petroleum / ethyl acetate (20:1) column chromatography, which showed: μH(400 MHz, CDCl3): 5.29-5.33(4 Hz, m), 1.63-1.48 (3H, m), 1.35-1,29 (17H, m), 1.20 (9H, s), 1,20-1.58(3H, m), 1.35 (18H, s). The product was cleared with colored petro-ethyl acetate (20:1) to yield a colored oil (R)-16-methoxyheptadec-8-1-yl pivalate (2.04, 0.0055.

(R)-16-methoxyheptadecyl pivalate (14)
Then the solution was filtered over a bed of celite and the solvent was evaporated for colourfree pivalate oil (R-16)-methoxyheptadecylpivalate (2.02 g, 0,00 546 mol, 99 percent). These oil were displayed as a colourless oil (r)-16-methoxyheptadecyl-pivalate (2.02 g, 0.00546 mol, 99 percent): Ţ H(400 MHz, CDCl3): 4.05 (2H, t, J 6.6 hz), 3.30-3.26 (1H, br, p, J 6.0, 5.8 Hz): 1.63-1.57 (8H, m),
(R)-16-methoxyheptadecan-1-ol (15)
The solution was then fitted with sat.aq sulfate at -20 ° C and the mixture was then added to a white ppt, THF added and then filtered over a bed of celite at room temperature for 30 minutes and solvent evaporated to provide the white solid (R)-16-methoxyheptadecan-1-ol (0.57 g; 0.00199 mol; 36%) shown; ̈H (400 MHz, CDCl3): 3.64 (2H, t, J 6.6 Hz), 3.31 (3H, s) The mixture has been then drained and the flask has been cleaned with gasoil, the mixture has been evaporated and distilled by chromatographic columns, eluting with petrol / ethyl-acetate(50:1) to produce a stable white (R)-1-bromo-16-methoxyheptadecane (0,48 g, 0,00138mol, 69%) which have been shown;δH (400 MHz, CDCl3): 3.41 (2H, t, J 6.9 Hz), 3.32 (3H, s), 3.30-3.24 (1H, br, p, J 5.9, 6.0 Hz), 1.89-1.82 (2H, p, J 6.9, 7.2 Hz), 1.45-1.40 (3H, m), 1.26 (21H, m), 1.13 (3H, d, J 6.1 Hz); δc (101 MHz, CDCl3): 77.2, 76.8, 55.9, 36.3, 34.1, 32.8, 29.7, 29.6, 29.5, 29.4, 28.7, 28.1, 25.4, 19.0; vmax/cm-1: 2913, 2848, 1469, 1086; [α]D23.8: -0.6° (c 1.67 g/mL, MeOH). The residue was then cleared through column chromatography. Petrol was used to first remove excess triphenylphosphine followed by DCM / methanol (10:1) in order to produce the white solid triphenyl phosphonium bromide (R)-(16-methoxyheptadecyl). (0.45 g, 0.000736 mol, 53%) which showed: δH (400 MHz, CDCl3): 7.86-7.77 (8H, m), 7.72-7.68 (6H, m), 3.80-3.73 (2H, m), 3.47 (1H, t, J 1.5 Hz), 3.30 (3H, s), 3.29-3.25 (1H, br, p, J 5.8, 6.0 Hz), 1.85-1.81 (5H,m), 1.63-1.60 (5H, m), 1.32-1.10 (24H, m), 1.11 (3H, d, J 6.1 Hz); δc (101 MHz, CDCl3): 134.9, 133.7, 133.6, 132.1, 131.9, 130.5, 130.3, 128.5, 128.4, 118.9, 118.0, 76.8, 55.8, 36.3, 30.4, 30.3, 29.7, 29.6, 29.5, 29.2, 25.4, 22.9, 22.6, 22.4, 19.0; vmax/cm-1: 2922, 2852, 1463, 1112, 772; [α]D22.4: -1.7° (c 1.81 g/mL, MeOH).
Conclusion
In summation, the synthesis of the target molecule was successful as demonstrated at the end of each experiment with both NMR and IR results. Sadly, there was relatively little yield for some reactions, which means that other synthesis methods needed to be examined. The synthesis of sulfone 3 would also be completed by reducing the ester to a sulfide, which would then be converted into an alcohol and then oxidized into a sulfone. The newly formed sulfone is then used with the next section of mycolic acid in another coupling reaction.
Reference
1. I. Smith, Clinical Microbiology Reviews, 2003, 16, 463-496.
2. S. Percival and D. Williams, Microbiology of Waterborne Diseases, 2014, 177-207.
3. E. Talbot and B. Raffa, Molecular Medical Microbiology, 2015, 1637-1653.
4. C. Collins and J. Grange, Encyclopedia of Food Sciences and Nutrition, 2003, 4067-4072.
5. M. Jackson, Cold Spring Harbor Perspectives in Medicine, 2014, 4, a021105-a021105.
6. Y. Vida, International Journal of Pulmonary & Respiratory Sciences, 2017, 1.
7. A. Vincent, S. Nyongesa, I. Morneau, M. Reed, E. Tocheva and F. Veyrier, Frontiers in Microbiology, 2018, 9.
8. P. Brennan, Tuberculosis, 2003, 83, 91-97.
9. H. Marrakchi, M. Lanéelle and M. Daffé, Chemistry & Biology, 2014, 21, 67-85.
10. M. Fraaije, N. Kamerbeek, A. Heidekamp, R. Fortin and D. Janssen, Journal of Biological Chemistry, 2003, 279, 3354-3360.
11. M. Beukes, Y. Lemmer, M. Deysel, J. Al Dulayymi, M. Baird, G. Koza, M. Iglesias, R. Rowles, C. Theunissen, J. Grooten, G. Toschi, V. Roberts, L. Pilcher, S. Van Wyngaardt, N. Mathebula, M. Balogun, A. Stoltz and J. Verschoor, Chemistry and Physics of Lipids, 2010, 163, 800-808.
12. J. Al Dulayymi, M. Baird, E. Roberts, M. Deysel and J. Verschoor, Tetrahedron, 2007, 63, 2571-2592.
13. M. Esmail, K. Astrofsky, C. Lawrence and F. Serluca, Laboratory Animal Medicine, 2015, 1015-1062.
14. M. Patterson and M. Fee, Laboratory Animal Medicine, 2015, 1109-1134.
15. C. Lee, H. You, J. Wang, Y. Tang and J. Liu, Journal of Clinical Microbiology, 2011, 49, 3096-3098.
16. H. Gebhardt, X. Meniche, M. Tropis, R. Krämer, M. Daffé and S. Morbach, Microbiology, 2007, 153, 1424-1434.
17. S. Pétursson, Journal of Chemical Education, 1997, 74, 1297.
18. B. Chatterjee, S. Bera and D. Mondal, Tetrahedron: Asymmetry, 2014, 25, 1-55.
19. J. Hanson, B. Dasher, E. Scharrer and T. Hoyt, Journal of Chemical Education, 2010, 87, 971-974.
20. H. Bestmann and R. Zimmerman, Comprehensive Organic Synthesis, 1991, 171-202.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



