Analyzing Paclitaxel in Plasma Using HPLC and SPE
Paclitaxel is a diterpenoid pseudoalkaloid compound that was isolated and extracted from the bark of the plant, Pacific Yew in around the year, 1960 [1]. It is widely utilized for the chemotherapeutic process to arrest the growth of tumour [2]. The robust and uncomplicated analytical technique for the extraction and measurement of this drug from the biological sample of human beings like plasma fluid is the High Performance Liquid Chromatography (HPLC) that is equipped with diode array detector (DAD) [3]. According to the scientific evidence following the validation of the assay procedure, the inter and intra accuracy along with the reproducibility of the assay technique was found to be appropriate with the recovery rate of the drug Paclitaxel within plasma fluid that was devoid of the drug was around 87%. The extraction process that will be utilized for the purpose is the Solid Phase Extraction (SPE) using extract-clean normal phase cyanopropyl silica cartridges for the clean up process of plasma fluid [3]. SPE is considered to be the most fast sample preparation technique before conducting the chromatographic analysis. As biological fluid like plasma is considered to be interference laden sample, the sample has to be diluted with buffer in a ratio of 1:1 [8]. The manipulation of the pH is considered to be critical step when dealing with ionisable compounds as the ionization sate of the compound can abruptly affect the characteristic of elution and retention. It is also required to centrifuge and filter the biological fluid as it may clog the column prior to the initiation of SPE. The strong ionic interaction of the salts present within the fluid with the analytes allows the use of high organic solvents for wash purpose and for the endogenous matrix displacement. In present days, unlike the binding and eluting procedure, the recent strategy to clean up the SPE prior to analysis with regard to majority of the matrix occurs by sorbent phase where packed SPE tubes with two layers of sorbent is used to get the highly purified eluate [8]. These strategies reduce the matrix effect during the procedure of SPE. Moreover, the sample will be loaded at a consistent but with reduced flow rate at about 1-2 drops per second to guarantee optimal retention. The wash step is considered critical for removal of the interfering substances that have been co retained along with the sample. Either 5 – 20% of methanol or pre treatment buffer can be used as wash solvents [8]. The graphical curve of calibration should be kept within the range of 10–500 ng mL−1. This methodology can be applied to estimate the value of the drug Paclitaxel within the blood plasma level of human samples. As per the scientific evidence the methodology worked well with the detection limit of the drug up to concentrations as low as 10 ng mL−1 [3]. If you're seeking healthcare dissertation help, these analytical methods can be crucial in supporting your research findings.

The HPLC system that can be utilized is an Agilent 1100 series apparatus set up with a quaternary pump, a column oven, a vacuum degasser, diode array UV detector (DAD), along with the HP workstation software for analysis [4]. The column that can be used is An Agilent Eclipse XDB-C18 column of length, breadth and diameter of 150 × 4.6 mm, 3.5 μm. There will be two mobile phase A and B where mobile phase A will be double distilled water, and mobile phase B will be acetonitrile and maintaining the flow rate of 1.2 ml/min. The samples will measured at the wavelength of 227 nm at 40° having the volume of injection set at 10 μl [4]. The program of the chromatogram will be set at –
Program Table of the Chromatogram:
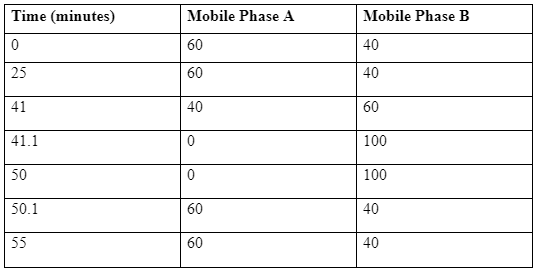
The standard solution preparation for the HPLC:
Accurately weighed quantities of drug Paclitaxel which is the reference standard will be dissolved within a mixture solvent of glacial acetic acid: methanol (1:200). Thereafter the solution would have to be diluted to obtain a standard concentration of 800 μg/ml [4].
The most important step of method validation will be conducted using the protocol of International Conference on Harmonisation Guideline on Validation of Analytical Procedures [5]. The standard solution will be a mixed one with five varied related components which will guide to assess the relative retention time (RRT) [6]. Moreover, a suitable solution to the system will be used to determine the selectivity of the methodology. The foremost breakdown products of drug Paclitaxel with or without emulsion such as 7-epipaclitaxel and10-deacetylpaclitaxel will be utilised as signal agents of the related compounds for product validation. A placebo solvent without emulsion and drug will be utilized for the examination of interferences, and along with that another examination will be used to ensure the selectivity is the degradation assay that will examine the samples at varied conditions of stress [6]. The detection of elutes with the aid of photodiode array will be used to comprehend the homogeneity and specificity of the peak achieved. The samples will be exposed to the varied stressful conditions such as acidic, base, oxidative and ultraviolet radiation (UV) to determine the purity of the peak obtained. All the related compounds with the drug Paclitaxel will be evaluated for measurement by recording the chromatogram, and the area under the curve or peak [6]. The peaks that will be achieved before and after the two breakdown products of drug Paclitaxel (based on the relative retention time) will not be considered, and the impurity percentage will be measured by using the formula: 100 (Fri/ru), where F denotes the relative response factor for each peak of impurity; ri refers to the area of the peak for each of the single impurity present within the sample solution; ru is the area under the peak achieved for the drug Paclitaxel [6]. Therefore, the summation of the percentages obtained for related substances was carried out to determine the total amount of the impurity present within the samples. Moreover, the accuracy of the extraction procedure or of the complete recovery will be determined by the comparative analysis with the contents of two compounds 7-epipaclitaxel and10-deacetylpaclitaxel which were mixed within the standard reference solutions and the placebo solution, at the percentage level of 0.5, 1 and 1.5 of 800 μg/ml. The procedural precision will be determined by repetition, the Limit of detection (LoD) along with the Limit of quantitation (LoQ) can be evaluated on a separate basis with the aid of signal to noise ratio (S/N) of 3 and 10, respectively. The parameter LoQ can be examined within the experimental process by the dilution of the standard solution of the drug Paclitaxel (Refer Fig: 1). It should be maintained with caution that the damage of the column due to oily matrix and other interferences can be prevented with the dilution of the solvents strategy [6].

Moreover the associated benefits with this analytical technique are the rapidity of the determination procedure with around screening of around 36 samples each day [3]. Another most important feature in relation with this technique is the cost effectiveness in comparison to the other mentioned strategies within the existing literatures and the parameters of validations are also observed to be extremely reasonable [3]. At last it can be mentioned that maximum of the analytical process for the measurement of any marker compounds within plant extracts or any biological sample is carried on by the process of HPLC which can determine the target compound along with the related ones with great precision level [7].

References:
- Wani MC. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325-7.
- Rowinsky EK, Donehower RC. The clinical pharmacology of paclitaxel (Taxol). InSeminars in oncology 1993 Aug (Vol. 20, No. 4 Suppl 3, p. 16).
- Caporossi L, De Rosa M, Pera A, Papaleo B. Simple analytical method for the determination of paclitaxel (Taxol®) levels in human plasma. Chromatographia. 2007 Dec 1;66(11-12):921-4.
- Xia XJ, Peng J, Zhang PX, Jin DJ, Liu YL. Validated HPLC Method for the Determination of Paclitaxel-related Substances in an Intravenous Emulsion Loaded with a Paclitaxel–Cholesterol Complex. Indian journal of pharmaceutical sciences. 2013 Nov;75(6):672.
- Guideline IH. Validation of analytical procedures: text and methodology. Q2 (R1). 2005 Nov;1:1-5.
- Rockville, MD. United States Pharmacopoeia. United States Pharmacopoeia Convention. 2004; pp. 1394–6
- Fu YJ, Sun R, Zu YG, Li SM, Liu W, Efferth T, Gu CB, Zhang L, Luo H. Simultaneous determination of main taxoids in Taxus needles extracts by solid‐phase extraction‐high‐performance liquid chromatography with pentafluorophenyl column. Biomedical Chromatography. 2009 Jan;23(1):63-70.
- Oh, J.H. and Lee, Y.J., 2014. Sample preparation for liquid chromatographic analysis of phytochemicals in biological fluids. Phytochemical Analysis, 25(4), pp.314-330.
Dig deeper into Analyzing Nice Guidelines on Surgical Site Infection with our selection of articles.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



