Investigating the Influence of Aspartame Concentration on Geyser Formation in Coke and Mentos Experiments
1. INTRODUCTION
The past few decades have seen an increase in access to information and flow of knowledge through the internet and social media. In terms of science, a plethora of common observations have been made regarding simple components that are easily obtainable. However, while some of these phenomena have been explained scientifically, others have remained shrouded in mystery. The experiment below was intended to formally investigate a common phenomenon; the formation of a geyser (violent explosion) when Mentos tablets are inserted in a Coke. Mentos are a brand of chewing gum common in the UK and Coke is the world’s most popular soft drink (Eichler, et al., 2007). This experiment was meant to investigate the effect of concentration of aspartame, on the amount of fizzing and gas produced when reacted with Coke. For students undertaking scientific studies, resources such as chemistry dissertation help can provide valuable guidance and support in exploring complex topics further.

Aspartame (APM), or N-L-alpha-Aspartyl-L-phenylalanine methyl ester (C14H18N2O5), is a sweet substance which is primarily used as an artificial sweetener in a plethora of food products including diet beverages, medicines, vitamins, yoghurts, desserts and chewing gum (Walters, 2001). The sweetener was accidentally discovered by James Schaltter in his endeavours to create a drug for gastric ulcer. The substance is described as a white, crystalline and odourless, with a very high amount of sweetness and minimal bitterness (up to 200 times sweeter than sucrose but has a slower onset) (Walters, 2001). Due to its high level of sweetness, less than 1% of aspartame is needed to achieve similar sweetness to that of sucrose. Moreover, the substance has a low calorie count thus is used in a lot of low calorie products. It provides 4 calories per gram. It is a derivative of aspartic acid (40%), Phenylalanine (50%) and methanol (10%) (Eichler, et al., 2007).
Although there are several descriptions of the supposed processes happening within the explosion, scientific proof of the process remains vague. For instance, while several scientific blogs report that the process may be as a result of a chemical reactions within the components of Mentos and Coke, others argue that the whole process is a physical reaction since no component is physically altered (Coffey, 2008) (see figure 1 below). The latter explanation has gained significant acceptance amongst various individuals in their quests to explain the reaction. In more details, it is argued that there is a decrease in the surface tension of the soft drink due to the pores of the coating of the mentos mint thus allowing the coke to form bubbles of the carbon dioxide gas much quicker (Loubière & Hébrard, 2004). I noted that although the theoretical explanations were viable, there still exists a significant amount of concern regarding the nature of the reaction.
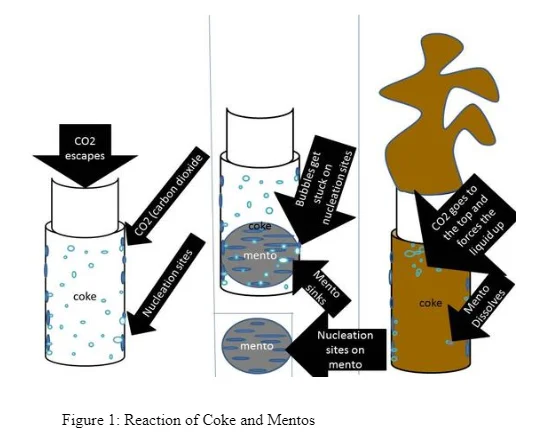
In this regard, this research was based on my personal fascination of the Coke and Mentos experiment and what reaction takes place in the bottle between the Coke and the Mentos in order for the violent explosion to take place. It involved an adaptation of the experiment, where a Mentos Sweet was dropped inside a mixture of carbonated water and aspartame solution. This study was based on the assumption that that the explosion is due to an increase in the nucleation sites of the reaction between the Mentos Mint the carbon dioxide in the beverage. Moreover, it was based on the assumption that the aspartame was the catalyst and therefore had an effect on the rate of the reaction.
2. RESEARCH HYPOTHESIS
The research was based on one hypothesis:
H1: An increase in the amount of sweetener will lead to a higher volume of gas.
The above hypothesis was based on the assumption that the sweetener will cause the gas to be
released quicker because the powder form of the sweetener will provide more nucleation sites for
the reaction therefore the reaction will be more vigourous and happen faster.
3. RESEARCH VARIABLES
According to Beaudry & Miller (2016), variables are those qualities, conditions, attributes and characteristics of subjects that can be observed or measured. They are divided into several categories which include control varies (those that cannot be altered in the course of the experiment), independent variables (those altered by the scientist) and the dependent variables (those that respond to the independent variable). In this experiment, the control variables are listed in the table below.
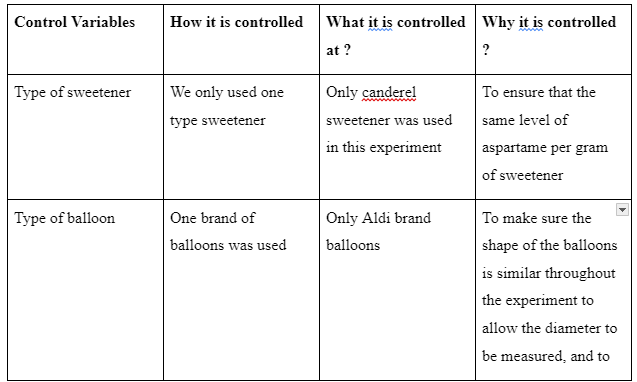
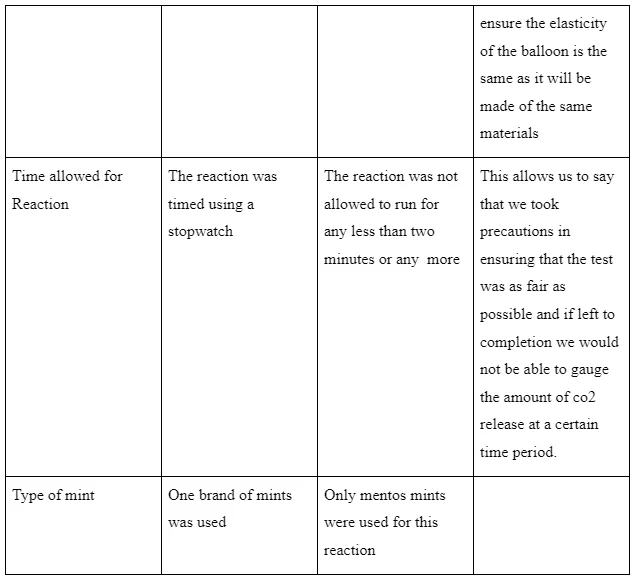
Table 1
The independent variable was the concentration of Aspartame sweetener added. The dependent variable was the volume of Carbon Dioxide gas released within two minutes.
4. MATERIALS AND METHODS
4.1 MATERIALS
The research utilised several materials and components to carry out the investigation:
- Aspartame sweetener (CANDEREL) 75g
- Mentos mints x 21 mints
- 21 Aldi© brand Balloons
- One Balance scale with an accuracy of +- 0.05 grams
- Pump
- 30 bottles of 500 cm3 Aldi carbonated water
- 5000 Cm3 Beakers
- Plastic container
- 2 Funnels
- 1 Stirring rod
- 1 stop watch
4.2 METHOD

- Weigh out 0.5 grams of aspartame sweetener (Canderel) in a weighing boat
- Pour the 0.5 grams of the weighed sweetener into the 500ml bottle of carbonated water and allow the sweetener to dissolve into the water.
- Place a mentos sweet in balloon
- Place the balloon over the mouth of of bottle so that the mouth is completely sealed and the Mentos Mint is in the water (use pump to stretch balloon if easier). This is shown in figure 2 below:
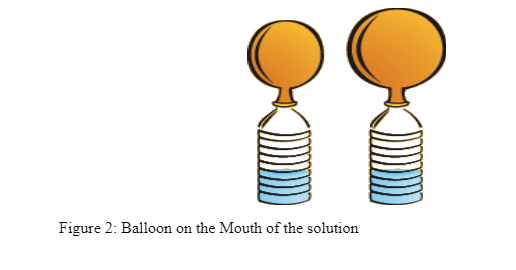
- Time for two minutes to allow for CO2 to flow into the balloon use a stopwatch in order to time
- After two minutes pinch the open end of the balloon to stop any CO2 from flowing out. (tie knot in balloon)
- Wrap a string around the balloon and measure length of the string in order to gage the diameter of the balloon
- Fill a bucket with water and place the bucket in a larger plastic box
- Place balloon on surface of the water
- Press balloon down with flat surface in order to push out water
- Measure the water that has come out using a measuring cylinder
- The volume of water in measuring cylinder will be the volume of the balloon
- Repeat for different concentrations of aspartame
5. RESULTS
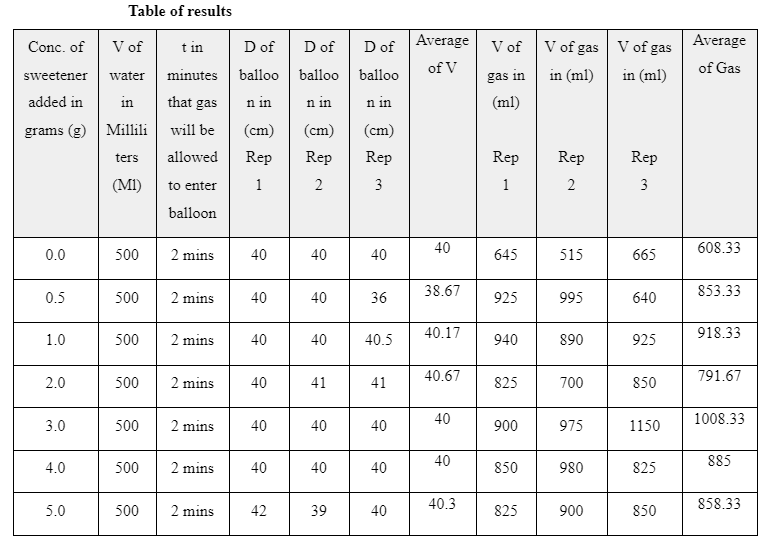
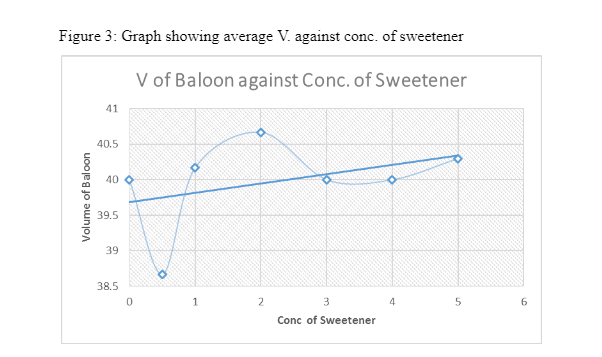
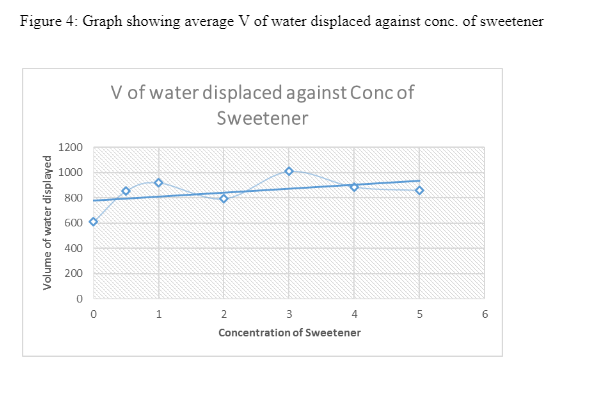
3. DISCUSSION
From the figures above, it is evident that the trendlines indicate a general increase in the volume of gas produced with increase in the amount of sweetener used. When the Mentos was introduced into the carbonated water, it had thousands of pores which reduced the polar-attractions between the H2O molecules (Eichler, et al., 2007). These pores are more in rougher Menots which lower the activation energy for the formation of Carbon dioxide bubble formations In this consequence, the number of nucleation sites for the gas-molecules increases (Loubière & Hébrard, 2004). As a result, bubbles of Carbon Dioxide increase and congregate. Their buoyant nature causes them to rise hence they form a geyser at the top of the bottle. The effects of aspartame in contributing to the increase in the Carbon dioxide can be explained by its ability to reduce the surface tension of the liquid and allow for rapid formation of bubbles. According to Xu (2009), surfactants can also have the same effect in lowering the surface tension of liquids to allow rapid bubble formation. When its concentration is increased, there is an increase in the amount of the surface tension lowered.
The eruption can therefore be termed as a purely physical reaction instead of a chemical reaction (Coffey, 2008). The addition of the Mentos sweet and the sweetener causes an increase in the rate of carbon dioxide produced. Once supersaturation is reached, the carbon dioxide accompanied by the liquid water shoots out of the bottle due to increased pressure (Eichler, et al., 2007). Although the process of nucleation can take place within any heterogamous surface, the rough surface of the Mentos physically increases the rate of the process.
7. CONCLUSION
From the results gathered, it is clear that the concentration of aspartame Sweetener in carbonated water did in fact have an effect on the rate at which carbon dioxide gas was produced. The Data show that compared to when there was no aspartame added to the carbonated water there was indeed an increase in the amount of CO2. At 0.0 grams (no sweetener), the average volume of Carbon dioxide gas produced was 608 cm3 while at 1.0 gram of sweetener the volume of gas Produced was 918 cm3. Although the process was not consistent, the trendlines in the graphs revealed a general increase in the volume of CO2.
However, the results revealed several weaknesses in the research procedure. From table 2 above, the results did not indicate a definite increase in volume. I noticed that the process of trapping the Carbon Dioxide in the balloon was not as smooth as theoretically expected. The volume of the balloon could also not be exactly determined as the balloons were not perfectly spherical. In fact, there was no sure means to know if the Carbon Dioxide volumes were a true reflection of the actual experiment. In this case, I believe that future researchers will have to alter the design of the experiment to ensure accuracy of results and precision of the values between various runs.
Dig deeper into Interprofessional Communication and Teamwork with our selection of articles.
References
- Beaudry, J. S. & Miller, L., 2016. Research Literacy: A Primer for Understanding and Using Research. New York: Guilford Publications.
- Coffey, T. S., 2008. Diet Coke and Mentos: What is really behind this physical reaction?. American Journal of Physics, 76(6).
- Eichler, J. F., Patrick, H., Harmon, B. & Coonce, J., 2007. Mentos and the scientific method: A sweet combination. J. Chem. Educ., Volume 84, p. 1120–1123.
- Loubière, K. & Hébrard, G., 2004. Influence of liquid surface tension (surfactants) on bubble formation at rigid and flexible orifices. Chemical Engineering and Processing: Process Intensification, 43(11), p. 1361–1369.
- Walters, D. E., 2001. Aspartame, a sweet-tasting dipeptide. [Online] Available at: http://www.chm.bris.ac.uk/motm/aspartame/aspartamec.html
- Xu, Q. et al., 2009. Effects of surfactant and electrolyte concentrations on bubble formation and stabilization. Journal of Colloid and Interface Science, 332(1), p. 208–214.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



