Assessing Staining Techniques in Haematology
Abstract
When examining the composition and structure of a tissue with aim to provide a diagnosis, the tissue is usually stained before the observation is conducted. To obtain an accurate, clear and conclusive observation, most tissues require the application of stains. This report aims to assess the application of the different stains used in haematology experiments including biology dissertation help. In the experiment, the periodic acid Schiff (PAS) stain method was applied to lamb liver tissues to detect glycogen and to distinguish it from other PAS positive elements in the section. The glycogen was identified by the magenta colour that was observed in the PAS staining
Moreover, the experiment enable key features that can be used to determine the condition of a liver to be observed. The periodic acid Schiff staining is used to identify the polysaccharides and glycoproteins present in the liver. However, in the PAS-D staining the diastase hydrolyses the polysaccharide present.

Introduction
Ordinarily, the stain that is used in haematology laboratories is the haematoxylin and eosin (H&E). It is normally used in the initial stages of an experiment to aid pathologists in obtaining a diagnosis. However, the H&E stain alone is not enough in many cases therefore it is complemented with other special stains to ensure a precise tissue diagnosis (Hauser, 2005). The term ‘special stain’ is used to refer to all other forms of stains other than H&E for example the PAS stain.
The periodic acid Schiff staining method is used to detect mucosubstance (such as glycoproteins and glycolipids) and polysaccharides. Glycogen is the main polysaccharide that is identified by the PAS staining method and it can be found in the skeletal muscles, kidney, and liver tissues of animals. The PAS staining method can be used in making therapeutic decisions in cases where a patient has lymphocytic leukaemia. In the diagnosis of the glycogen storage disease, diastase (α-amylase) digestion procedure together with the PAS staining method is used (Carson & Hladik, 2009).
The first reaction that occurs is the oxidizing of the carbon-to-carbon bonds between the two adjacent hydroxyl groups in molecule. Molecules that contain glycol groups or their alkylamino or amino derivatives will be oxidized by the periodic acid to form a dialdehydes, which are Schiff reactive therefore reacting with Schiff’s reagent (which contains a mixture of sodium metabisulphite, basic fuchsin and hydrochloric) to form an insoluble magenta compound. The concentration of the hydroxyl group presents often determines the intensity of the colour observed in the compound (Carson & Hladik, 2009). Contrarily, diastase breaks down the products that originate in the tissue polysaccharides i.e. starch and glycogen.
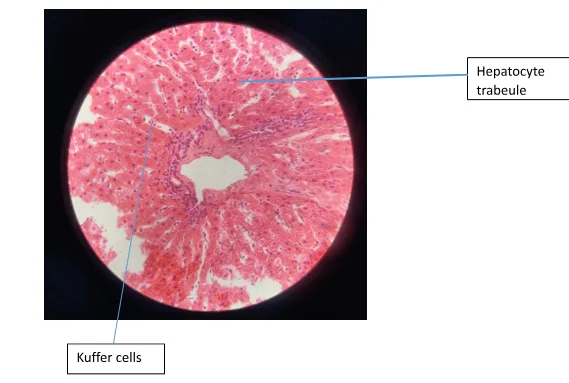
The liver being the main tissue of study in this experiment, contains lobules that are structural and functional units. The hepatocyte (the liver parenchymal cell) is a polygon cell with a central nucleus. These cells are one cell thick with sinusoid on each side and are arranged in plates. The sinusoids are lined by the fenestrated endothelial cells while the Kupffer cells are located on the luminal side of the sinusoid. The hepatic cells are located in the space of the Disse and ae presinusoidal pericytes. The cytoplasm contains a lot of glycogen and is granular as well as eosinophilic (Hauser, 2005).
This experiment aims to detect glycogen present in a section of lamb liver and to distinguish it from other PAS positive elements.
Materials, experimental outlines and rationale
The tissue used in the experiment was paraffin embedded lamb liver sections at 4-5μm. The solutions and reagents used were: α-amylase/diastase, 0.5% periodic aid solution, Schiff reagent and Mayer’s Haematoxylin solution. Other reagent used in the experiment were xylene and ethanol.
Prior to the beginning of the experiment, the liver sections were de-paraffinized in 2 changes of xylene for 5 minutes each, in the hood. Thereafter, the sections were rehydrated in absolute alcohol for a period of 3 minutes each. Furthermore, rehydration was continued using 90% of alcohol for 3 minutes and 70% of alcohol for 3 minutes. The sections were then brought to distilled water.
The sections that required amylase digestion, were treated with a solution of amylase for a period of 20 minutes and then washed under running tap water. The slide was first washed using dH2O for 30 seconds and the excess dH2O was tapped off before the periodic acid (0.5%) was applied for 5 minutes. Using a small piece of tissue the excess solution was tapped off and the slide was then put in the Coplin jar and rinsed for 3 changes of dH2O, for a period of 1 minutes each.
The Schiff’s reagent was added on to the slides after the excess water was wiped off and the colour change was observed. After the Schiff’s reagent was wiped of the slide, it was placed in the Coplin jar with warm water from the tap, and later changed with fresh water after 1 minute. Counterstaining was done for 8minutes using haematoxylin after the water was tapped off. Subsequently, before washing the slides the haematoxylin was tapped off. The slides were then place in the Coplin jar in running water for a duration of 3 minutes.
The sections were then dehydrated through 70% of alcohol for 2 minutes and 90% of alcohol for 2 minutes in the hood. This process was done for 2 changes of absolute alcohol for 2 minutes each. Thereafter, the sections were cleared in 2 changes of xylene for 2 minutes each and the slides were attached with xylene-based mounting medium and coverslip. Using a light microscope, the structure of the tissue was observed and the images were captured.
Methodology adapted from: Chapter 8 (Histochemistry: the general considerations and histochemistry of carbohydrates) in Cellular Pathology, by D, J Cook 3rd Edition.
Results:
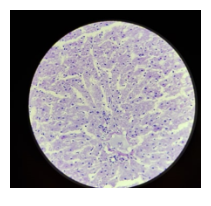
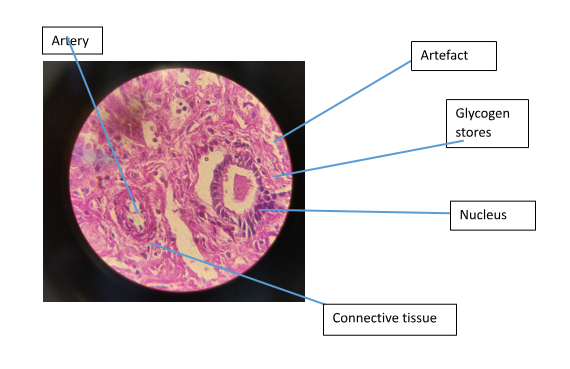
Figure 1 shows hepatocyte trabeculae separated by sinsusoidal spaces. The hepatocytes with an extensively smooth and rough endoplasmic reticulum can be seen. In Figure 4. The artery can be seen and it has been surrounded by a mass of connective. In the section stained with PAS-D there was no magenta colour observed (Figure 3).
Discussion & Conclusion
Upon the general observation of H&E stains, sharply defined plasma membranes have been identified (Figure 1). The H&E stain aid in identifying the cytoplasmic inclusions and nucleus. A large quantity of the mitochondria can be found in the hepatocytes. This together with the smooth and rough endoplasmic reticulum contribute to the eosinophilic staining of their cytoplasm (McAdams & Wilson, 1966).
To demonstrate the presence of true glycogen PAS-D is used. The amylase breaks hydrolyses the glycogen into smaller sugar molecules (glucose and maltose) which can be easily washed out. Areas that are observed to be PAS positive (Figure 4) and that are PAS negative in the digested section (Figure 3) represent true glycogen (McAdams & Wilson, 1966). However, in the case where the PAS positive is observed in both the treated and untreated sections the glycogen hasn’t been broken down by the enzyme. The reason for using the enzyme is to insure that glycogen can be differentiated from other PAS positive elements present in the sample such as mucin.
It is crucial to ensure that the periodic acid isn’t diluted, the Schiff’s agent is stable and the enzyme being used is correct to avoid false positives. Dialdehydes (e.g. glutaraldehyde) shouldn’t be used as a tissue fixative as they can react reagent and give a false positive PAS staining (Cook & Warren, 2015).

In conclusion, the PAS staining and the digestion are essential in the identification of glycogen. The digestion step is crucial as it helps in identifying the presence of glycogen. PAS staining help pathologist identify the quantity of glycogen and through the patterns observed glycogen storage diseases can be identified.
Looking for further insights on Cognitive Bias in Expert Testimony? Click here.
References
- Carson, F. L., & Hladik, C. (2009). Histotechnology: A self-Istructional Text (3rd ed.). Hong Kong: American Society for Clinical Pathology Press.
- Cook, D. J., & Warren, P. J. (2015). Histochemistry: The general considerations and histochemistry of carbohydartes . In Celluar Pathogy (pp. 131-156). Banbury: Scion Publishing Limited.
- Hauser, S. C. (2005). Mayo Clinic Gastroenterology and Hepatology Board Review. CRC press.
- McAdams, A. J., & Wilson, H. E. (1966). THE LIVER IN GENERALIZED GLYCOGEN STORAGE DISEASE . The Children's Hospital and The Children's Hospital Research foundation, 99-111.
Dig deeper into Assessing Antiviral Therapies for COVID-19 with our selection of articles.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



