Navigating Breast Cancer Research: A Methodological Journey through CASP Framework
Methodology:
Several high impact peer-reviewed journals have been studied via comprehensive search method and the approach for the evaluation of the literature review has been implemented. The Critical Appraisal Skills Programme (CASP) methodology framework developed in Oxford in 1993 by Dr. Amanda Burls (Singh, 2013), has been applied for the critical appraisal of the literature search evidences. Following steps were followed in the methodology section of the study to conduct a systemic review for literature review dissertation help:
1. Recognizing the research question.
2. Selection of the relevant works of literature
3. Charting of the data of the selected evidence.
4. Pooling, summarising and reporting the data.
Recognizing the research question
The study was conducted with a broad definition for the study population, results, interventions so that a detailed coverage can be obtained in the search strategy with the clearly defined concept was maintained which could be continuously refined based on the number of references and scope, parameters are set. Colquhoun, et al, 2014 have established the idea through necessary observations and stated that required steps could help in making the research clarifications and in linking the review results with the assigned research questions.

What is breast cancer and its prevalence among UK residing women population?
What are the risk factors associated with breast cancer?
What is the role of a family towards a breast cancer patient?
What are the emotional and psychological crises faced by cancer affected mother?
What is the role of a father in a family and how role reversal occurs?
What is the psychological impact of breast cancer upon family and how the family copes up with the trauma?
What should be the approach of nurses towards a family having a breast cancer patient?
Selection of the relevant works of literature:
Primary studies (including both published data and unpublished studies are included) and reviews are selected based on the best understanding of the central research question. Therefore, a strategy was adopted where research evidence was searched from different sources. Suitable sources were identified after reviewing multiple electronic databases which comprises a huge number of high impact peer review contents such as Cumulative Index to Nursing and Allied Health Literature, PsycINFO, PubMed, Google Scholar, Medline, Taylor, Karger, Plos-One and Francis Online. Grey literature databases, utilised by the researcher for searching scientific contents are GreyNet International, Open Grey and Med Nar. The search was done with restriction to the most updated contents up to the year 2019 so that relevant and latest content can be available for the literature review purpose. One particular limitation was faced by the researcher was the non-availability of the full-length document apart from the abstract due to the registration service particulars of the University. All the in-text references of the resulted papers were also reviewed to analyse and evaluate the content of the relevant databases.
Study selection:
The inclusion and exclusion criteria are developed once the research idea becomes familiar with the literature search. To define the inclusion criteria the title of the article and abstract should contain the keywords or the definite search item. Relevant search should be done to match the requisite of research question though it may not contain the keywords or the search items within the title or abstract of the articles. To define the exclusion criteria, any studies that are falling “out of scope” for the review and not matching with the set research questions will not be considered. Any literature not written in the English language will not be considered for the review purpose. Therefore all the pieces of literature based on the defined inclusion and exclusion criteria are considered for the literature review.
Research philosophies can be of two types: Positivism and Interpretivism. Here the use of Positivism research philosophy will be more appropriate which states that the analysis and interpretation of the research data could become successful through the acquired truth and evident facts from authenticated sources. This particular approach allowed the researcher to develop an analytical mind to investigate about the concerned research problem. A structured and quantitative research approach was applied for solving the research problem (Williams et al. 2017). Systemic review approach has been referred as the process for the purpose of literature review. It is done by applying systematic method process such as critical appraisal research studies, analysis of the secondary data, and the data that had been produced quantitatively and qualitatively (Saunders et al. 2018; Bettany-Saltikov and McSherry, 2016). This particular approach has been used to obtain a detailed and exhaustive analysis of all the available literatures on the electronic databases based on the inclusion criteria. The benefits associated with this method of review is that it uses scientific methods for identifying and selecting the research which in turn reduces any form of bias in the study along with the productions of accurate and reliable results and conclusions in relation to the research hypothesis of the study. The researchers can study a wide number of literatures which helps them to evaluate the fact properly and present them in an accurate manner (Patterson et al. 2016). There are few drawbacks of using this approach by the researcher which includes that the researcher may find it difficult to compile the results at the end of the study as different researchers used different approaches to conduct their study (Rudmik and Soler, 2015). Therefore the researcher has to be very attentive while compiling the results from a wide range of studies to ensure the accurate validity of the results. The title of the research articles should match with the research questions and to ensure the accuracy the abstract has to be studied. If the abstract seems to be suitable with the research hypothesis of the study, the entire full length study will be done to obtain a quality appraisal. All the evidence will be included in the CASP flowchart in terms of retrieved, included and excluded studies (Lewis et al. 2019; Liberati et al, 2009).
Keywords:
Breast cancer, prevalence, families, children, father, husband, spouse, daughter, son, psychological problem, anxiety, stress, depression, worries, strain, tears, concerns, problems, cancer affected mother, social embarrassment, compromised ability .
Database search:
Cumulative Index to Nursing and Allied Health Literature, PsycINFO and Medline are the most searched databases by the researcher concerning this topic. The strategy used for search involves the medical subject heading (MESH) and it has been applied to get reliable results. The researcher used strategies such as “truncation search” in addition to asterisks for the words such as breast cancer*, psychological impact*, emotional crisis *, compromised ability *, cancer affected mother *, social embarrassment* etc. This particular approach has helped the researcher to obtain a wide range of information from several database sources. Again the search outcomes were enlarged with the application of Boolean operators for example ‘AND’, ‘OR’ for tapering down the search results.
Study design:
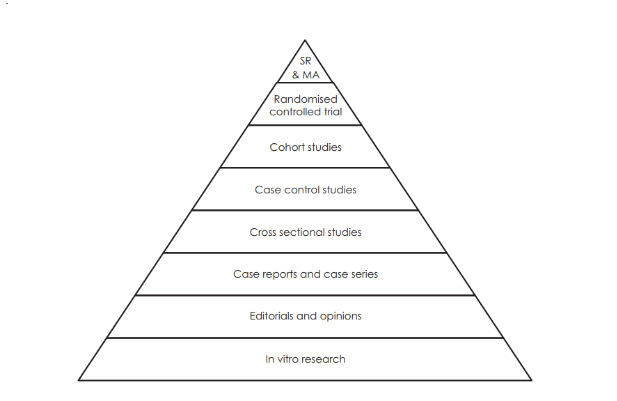
To understand the type of the study conducted by the researcher is essential to ensure the quality of the evidence provided by the researcher. The knowledge of the study design will help the researcher to determine the study, its strength and weakness. The primary research study can be both experimental and observational. The randomised control trials (RCT) is considered to provide the strongest evidence with respect to the decision making in the clinical aspects and the study design may also reduce the chances of research bias. Case studies and case reports are very common descriptive studies but they are not designed to test the association between two factors such as a therapy and a treatment (Refer Fig: 1) (Calzetta, 2017; Sabaté, et al, 2009).
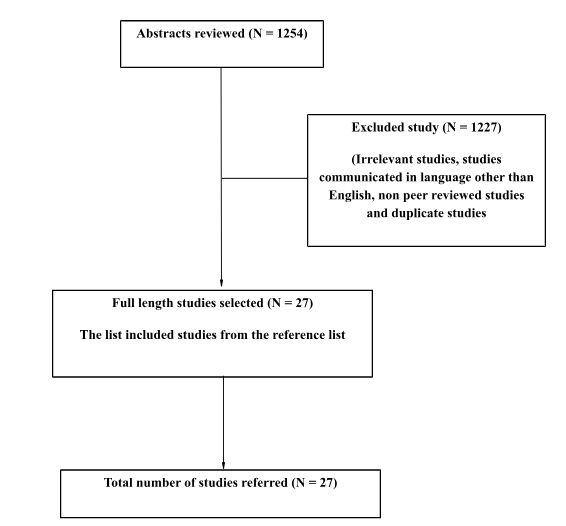
Search outcomes:
Detailed searching of all electronic databases based on the inclusion and exclusion criteria resulted from about 1254 research evidence outcomes (journal articles and medical reports) relating to the title of the research. The reason behind the adoption of the strategy was to maintain the accuracy level in the search outcomes and removal of any studies which do not match with the context of the research by applying the Critical Appraisal Skills Programme (CASP) methodology framework as illustrated via a flow chart. (Refer Fig 1).
Analysis of the data:
The analysis of the data is considered to be a challenging task as it is used to assess the quality of the study. The data will be analysed using the Quality Appraisal Tools (QAT) as suggested by Voss and Rehfuess, 2013. There are varieties of Quality Appraisal Tools available which may be used to facilitate the process. For the present study the data analysis will be done based on the Critical Appraisal Skill Programme (CASP) to ensure a qualitative research. This particular tool helps to conduct critical analysis in a wide variety of settings which includes the public health. This is a set of 8 appraisal tool checklist based approach designed to be used in an educational programme or in a workshop setting. This particular quality appraisal tool is used to analyse the Randomised Controlled Trials (RCT), Systematic Reviews, Case Control Studies, Cohort Studies, Economic Evaluations, Qualitative studies, Diagnostic Studies, and Clinical Prediction Rule. The website of the programme is (www.casp-uk.net) and it is a virtual platform. The tool helps in finding the right information by highlighting the searches from various types of sources which includes the secondary sources, bibliographic sources and the gray literatures. The appraising section of the tool focuses mainly on the validity and reliability of the scientific papers, the significance of the outcome and the application of the findings to the concerned research project. There are several standardised checklist available on this webpage and this can be downloaded. The section that deals with the “acting on the evidence” focuses on the application of the data findings to our own research hypothesis, thinking about the practicality of the issues which may affect our study. The patient centred study is also explained and provides evidence to explore that context of the research topic.
There is a separate checklist which consist of about 10 -12 questions are available for the Randomised Controlled Trials (RCT), Systematic Reviews, Case Control Studies, Cohort Studies, Economic Evaluations, Qualitative studies, Diagnostic Studies, and Clinical Prediction Rule study. The checklists are wide ranging which can give direction to any researcher and can also guide on the outcome of the study. The CASP guidelines are very easy to follow even by a junior researcher.
There are two checklists namely the Diagnostic Checklist and the Economic Evaluation Checklist. STARD stands for Standards for the Reporting of Diagnostic Accuracy Studies is the most prevalent checklist available in the website www.stard‑statement.org offers a detailed flowchart and checklist. But the use of CASP diagnostic Checklist consisting of 12 questions is much easier and also simple to follow. CASP economic evaluation checklist provides an easy guideline to evaluate the economic benefits of the study.
The CASP works on the basis of a systemic process due to which the strengths and weakness of any study can be identified. It also guides the researcher in the study design approach and regarding the application of the study in the local context in a most cost effective way. Study designs are exposed to a wide range of variables that develops bias in the study design and therefore it is recommended to use standard lists of checklist to ensure the quality of the study undertaken. Sticking to the guideline of CASP enhances the significance of the study along with its findings (Singh, 2013)
Internal validity of the data:
Internal validity also measures the quality of the data as it is examines the study population. It asses the study based on several questions such as: did the researchers perform the study properly? After determining the research hypothesis or topic and the best study design the methodology section should be critically analysed to ensure the quality and strength of the evidences provided in the study. This highlights that whether the potential bias in the research has been addressed or not, whether the study had been conducted based on the original protocol and the findings had been analysed statistically or not. Few evidences will present very little information on the topic which could hardly match with the reality and the interpretation of that type of the study should be done with caution (Pinchbeck, 2020).
External Validity of the study:
After determining the internal validity of the study the next step of the study is to determine the external validity. External validity determines the validity of the study when the results or findings of the study are applied to the outside populations or the targeted populations, i.e., the targeted patients. In this case the study population is the women population who have children and are suffering from any stage of breast cancer. It is also advisable that the researcher should determine that whether the outcome of the study is of any relevance to the targeted patient populations and / or any outcome of clinical importance had been ignored or not. Moreover, a study can be externally valid only if it is internally valid (Pinchbeck, 2020).
Risk of Bias:
For conducting a systemic review research the risk of bias had to be evaluated for the studies included for the purpose. It is defined as the risk or the “a systemic error or deviation from the truth in the result or inference” of the study. It is considered to be replaceable with the internal validity of the study and therefore is defined as “the extent to which the design and conduct of a study are likely to have prevented bias”. Different tools are available for the assessment of risk bias of the study which includes Cochrane tool, ROBINS-1, ROBINS-E other than the critical appraisal skill programme (CASP), Scottish Intercollegiate Guidelines Network (SIGN) Systematic Reviews and Meta-Analysis Checklist, JBI (Joanna Briggs Institute) critical appraisal instrument for Systematic reviews and Research Syntheses . The tools examine about error in the study design and also examine the outcome to assess the possible bias in the study. To examine about the error in a randomised control study (RCT), development of the guideline and systemic review research Cochrane tool is applied whereas in case when an observational study of an intervention is compared with hypothetical randomised control study the analysis of the data is carried on by ROBINS-I tool (Sterne et al, 2016; Savovic et al 2014; Higgins et al, 2011). ROBINS-I was adapted in place of ROBINS-E where the term “intervention” has been replaced by “exposure”. The tool examines about the five different domains of bias such as selection, performance, attrition, reporting, and other. The CASP tool was used to analyse the validity and quality opf the evidences and the research bias was measured with the help of the CASP along with SIGN.
Other factors to consider:
The findings of the present study should be correlated with the outcomes of the previous studies, i.e., whether the findings are consistent or contradictory to the findings of the similar studies conducted in the past (Pinchbeck, 2020).
Statistical Validation:
The author should be able to understand and critically evaluate any types of statistical analysis of the findings. But if the authors had used any uncommon statistical tests then they should provide justification for the use of such tests in detail along with a reference. For conducting a RCT study the calculation of a sample size is crucial and should be justified using appropriate statistical calculations. The statistical method employed to compare between two sets of data should be clearly defined along with the assumptions. The result section should clearly mention the tests used and their justifications so that the readers may understand that how the author have compiled the findings (Pinchbeck, 2020).
Ethical considerations in the study:
The researcher performing the systemic review should declare about the potential conflict of interests and all the authors of the included study would need to be accredited to avoid any forms of the act of plagiarism (Wager and Wiffen, 2011). The ethical principles concerning the beneficence and malfeasance should be considered by the primary researcher after obtaining the informed consent from all the probable participants which will lower down the risk of any damage to them and also maintain the confidentiality of the candidate while reporting the study. It also takes care about the use of any kind of deceptive practices along with the right to pull out from the research background. Since the current study is a systemic review also termed as secondary research therefore there is a very limited role of ethical considerations in the study. Considering the ethical evaluation of the study the methodology of a systemic review based research can be improved to ensure a better quality of the work (Schulz, Moher, Sand Altman, 2001; Moher et al 2009; Stroup et al 2000). It is also essential to evaluate the findings of the existing work from the website based on the access available to the restricted published data. As there is no funding is available for the present study therefore the rule to declare about the funding agency of the study is not applicable in this case (Higgins and Green, 2011).
Limitations of the study:
As there is no funding agency available to conduct the study therefore the limitations faced by the researchers is to access the restricted journals and published data available on the electronic database relevant to the search topic. Moreover, the author may access the University library to access those evidence depending upon the registration of the University. The limitation to time can be managed by planning the study within the time allocated.

Conclusions:
At last it can be concluded that it depends upon the researcher to decide the reliability of the results presented in a paper and whether that data could be used to provide better care service to the patients. There are no single critical appraisal tools that can be applied in all types of the study but there are several checklists available online that can guide the researcher to understand that whether the paper had followed the specific criteria that has to be employed in an epidemiological study.
References:
Colquhoun, H.L., Levac, D., O'Brien, K.K., Straus, S., Tricco, A.C., Perrier, L., Kastner, M. and Moher, D., 2014. Scoping reviews: time for clarity in definition, methods, and reporting. Journal of clinical epidemiology, 67(12), pp.1291-1294.
Singh, J., 2013. Critical appraisal skills programme. Journal of pharmacology and Pharmacotherapeutics, 4(1), p.76.
Williams, L., Rycroft‐Malone, J. and Burton, C.R., 2017. Bringing critical realism to nursing practice: Roy Bhaskar's contribution. Nursing Philosophy, 18(2), p.e12130.
Saunders, B., Sim, J., Kingstone, T., Baker, S., Waterfield, J., Bartlam, B., Burroughs, H. and Jinks, C., 2018. Saturation in qualitative research: exploring its conceptualization and operationalization. Quality & quantity, 52(4), pp.1893-1907.
Bettany-Saltikov, J., Kandasamy, G., Van Schaik, P., McSherry, R., Hogg, J., Whittaker, V., Arnell, T. and Racero, G.A., 2019. School‐based education programmes for improving knowledge of back health, ergonomics and postural behaviour of school children aged 4–18: A systematic review. Campbell Systematic Reviews, 15(1-2), pp.1-11.
Rudmik, L. and Soler, Z.M., 2015. Medical therapies for adult chronic sinusitis: a systematic review. Jama, 314(9), pp.926-939.
Lewis, J.M., Feasey, N.A. and Rylance, J., 2019. Aetiology and outcomes of sepsis in adults in sub-Saharan Africa: a systematic review and meta-analysis. Critical Care, 23(1), p.212.
Liberati, A., Altman, D.G., Tetzlaff, J., Mulrow, C., Gøtzsche, P.C., Ioannidis, J.P., Clarke, M., Devereaux, P.J., Kleijnen, J. and Moher, D., 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine, 151(4), pp.W-65.
Calzetta, L., Roncada, P., di Cave, D., Bonizzi, L., Urbani, A., Pistocchini, E., Rogliani, P. and Matera, M.G., 2017. Pharmacological treatments in asthma‐affected horses: a pair‐wise and network meta‐analysis. Equine veterinary journal, 49(6), pp.710-717.
Sabaté, D., Homedes, J., Salichs, M., Sust, M. and Monreal, L., 2009. Multicentre, controlled, randomised and blinded field study comparing efficacy of suxibuzone and phenylbutazone in lame horses. Equine veterinary journal, 41(7), pp.700-705.
Pinchbeck, G.L. and Archer, D.C., 2020. How to critically appraise a paper. Equine Veterinary Education, 32(2), pp.104-109.
Sterne, J.A., Hernán, M.A., Reeves, B.C., Savović, J., Berkman, N.D., Viswanathan, M., Henry, D., Altman, D.G., Ansari, M.T., Boutron, I. and Carpenter, J.R., 2016. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. bmj, 355, p.i4919.
Savović, J., Weeks, L., Sterne, J.A., Turner, L., Altman, D.G., Moher, D. and Higgins, J.P., 2014. Evaluation of the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Systematic reviews, 3(1), p.37.
Higgins, J.P.T., Altman, D.G. and Sterne, J.A.C., 2011. on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. Cochrane handbook for systematic reviews of interventions version, 5(0).
Wager, E. and Wiffen, P.J., 2011. Ethical issues in preparing and publishing systematic reviews. Journal of evidence-based medicine, 4(2), pp.130-134.
Higgins, J.P., Altman, D.G., Gøtzsche, P.C., Jüni, P., Moher, D., Oxman, A.D., Savović, J., Schulz, K.F., Weeks, L. and Sterne, J.A., 2011. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj, 343, p.d5928.
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., Shekelle, P. and Stewart, L.A., 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews, 4(1), p.1.
Higgins, J.P., Altman, D.G., Gøtzsche, P.C., Jüni, P., Moher, D., Oxman, A.D., Savović, J., Schulz, K.F., Weeks, L. and Sterne, J.A., 2011. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj, 343, p.d5928.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



