Adiponectin Suppresses Human Pancreatic Cancer Growth
Introduction
Cancers are defined as a group of diseases caused by abnormal cell proliferation with a characteristic of invading new cells and tissues. There are different types of cancers, and every human organ or tissue seemingly has a type of cancer that can potentially affect it. One of the most fatal cancers is pancreatic cancer with a relative survival rate of less than 8% in 5 years on average (Siegel, et al., 2016).

It is estimated that pancreatic cancer deaths has reached 330,000 globally (Torre and Bray; Siegel, et al., 2012), but this number may even be higher putting into consideration cases that were not reported from remote areas. In China, pancreatic cancer already ranks as the sixth most common cause of death from cancer (Chen and Zheng; Baade, et al., 2015; Clin, 2016), and ranks third most common cause in the United States (Siegel, et al., 2016). The pathogenesis of pancreatic cancers is still insufficiently known, causing a lack of effective treatment for this deadly disease that has claimed nearly half a million people. The authors of this paper of choice, deemed it urgent to accelerate research underlying the development of pancreatic cancer (Jinghui, et al., 2019).
Cancers have a wide range of causal agents from chemicals such as industrial solvents, diet for example red meat, infections, radiations such as repeated exposure to X-rays, heredity, physical agents such as heavy metals, hormones, autoimmune disorders and lifestyle diseases such as obesity and diabetes. There is sufficient evidence to support that obesity is associated with an increased risk of various cancers, including pancreatic cancer (Loomis, et al., 2016; Moghadam, et al., 2013; Jinghui, et al., 2019). Previous researches have demonstrated adverse associations of obesity with survival among pancreatic cancer patients (Courneya, et al., 2014) and evidence has showed that adipokines secreted by adipose tissue are closely involved in obesity-related cancers (Nam, 2017).
Adipokines are cytokines involved in cell signaling pathways. The first adipokine to be discovered was leptin in 1994. Adiponectin, an hormone involved in regulation of glucose and fatty acid metabolism is one of the major adipokines secreted by mature adipocytes and also plays a vital role in modulating inflammatory processes (Minokoshi, et al 2002; Cheng et al., 2012). It has been shown that serum levels of adiponectin is significantly reduced in obese individuals (Lu, et al., 2003).
The aim of the authors was to investigate the functional role of adiponectin in pancreatic cancer as well as its underlying molecular mechanisms. In this study, they investigated the influence of adiponectin on human pancreatic cancer via a series of in vitro and in vivo experiments (Jinghui, et al., 2019).
Methods
The following procedures were conducted by authors to arrive at their objectives,
Cell culture and treatment
Human pancreatic cancer BxPC-3 cells and CFPAC-1 cells were maintained in RPMI 1640 and IMDM containing 10% FBS respectively. Cells were incubated in equipment at a constant temperature and humidity with 5% carbon dioxide in air. The cells were treated with recombinant human adiponectin and then subjected to further analysis. To detect the protein phosphorylation level of Akt and GSK-3β, BxPC-3 cells were treated with or without adiponectin in the absence of serum. The cells were then lysed in RIPA buffer western blot analyses. To identify the role of GSK-3β, cells were treated with TWS119, a specific GSK-3βinhibitor in the absence or presence of adiponectin. To determine whether proteasome mediated the effect of adiponectin on β-catenin, cells were pre-treated with proteasome inhibitor MG-132, followed by adiponectin treatment.
Generation of stable adiponectin overexpressing cells and AdipoR1/AdipoR2-knockdown cells
The full-length human ADIPONECTIN gene was inserted into the expression lentivector pCDH-CMV-MCS between the NheI and BamHI sites. Resulting recombinant gene was used to infect BxPC-3 cells or CFPAC-1 cells to generate stable adiponectin overexpressing pancreatic cancer cells (BxPC-3-adiponectin or CFPAC-1-adiponectin).
The control cells (BxPC-3-VC cells or CFPAC-1-VC cells) were generated by transduction with an empty virus. The gene specific targeting sequence for RNAi-mediated knockdown ofAdipoR1 (5’-GCAAAGACTATGATGTTAA) or AdipoR2 (5’-GTGTAGAAGTTGAGAAGAA) was inserted into the shRNA expressing lentivector pGreenPuro. The corresponding pancreatic cancer cells were transduced with a recombinant lentivirus carrying the counterpart scramble sequence (scRNA) to generate the control cells (BxPC-3-scRNA cells or CFPAC-1-scRNA cells).
Cell proliferation assay
Cells were plated in a 96-well plate and after attachment to the wall, the cells were cultured in FBS-free medium then treated with or without adiponectin in the corresponding culture medium containing 10% or 2% FBS. At 0, 24, 48 and 72h after treatment, the culture medium was replaced by a fresh medium containing CCK-8 reagent, followed by incubation at 37°C. Absorbance was measured at a wavelength of 450 nm using a microplate reader.
Western blot
Cells were scraped into lysis buffer, and lysates were then quantitated using a BCA Protein Assay kit. Equal quantities of proteins were added to the gel for electrophoresis and transferred to polyvinylidenedifluoride membranes. Various primary antibodies were used to determine the expressions of target proteins, including adiponectin, AdipoR2, TCF7L2, cyclinD1, GSK-3β, pGSK-3β (Ser9), Akt, p-Akt (Ser473), AdipoR1, β-catenin, cyclinA2, cyclinE1, p21 and β-actin. The protein-antibody complexes were detected using a Pierce Super Signal West Pico chemiluminescent substrate. Bands of interest were quantified by densitometry using Image J software.
Xenograft model
Animal experiments were performed according to the protocols approved by the Medical Experimental Animal Care Commission at the Shanghai Cancer Institute. The male athymic BALB/c nude mice (5-6 weeks old) used in this study were provided free access to food and water. Mice were randomly assigned to two groups, and subcutaneously inoculated with BxPC-3-scRNAcells or BxPC-3 siAdipoR1/2cells (1×107 cells/mouse) in the flank region. The tumor size was measured weekly starting 2 weeks after inoculation.
Immunohistochemical analysis
Tumor tissues were fixed in 10% buffered formalin, embedded in paraffin, and sectioned at 5 μm. Sections were incubated with the anti-PCNA antibody. PCNA staining was visualized using the ImmPress HRP Reagent kit with NovaRed as a substrate. Hematoxylin was used as a counterstain for the cell nucleus. The authors counted the number of total cells and PCNA-positive cells per 400× microscope field with 3 random fields per mouse.
Cell cycle analysis
After being treated with adiponectin, BxPC-3 or CFPAC-1 cells were trypsinized and then washed with PBS. The cells were then transferred into ice-cold ethanol (70%) followed by staining with propidium iodide containing RNase A. The cell cycle was analyzed using FACSCelesta flow cytometer, and the percentages of cells in G0-G1, S, and G2-Mphasewere evaluated using the software FCS Express 4.0.
Quantitative real-time PCR
Total RNA was extracted with TRIzol, and cDNA was prepared by using a FastQuant RT kit Quantitative real-time PCR analysis was performed on a StepOne Plus real-time PCR system. The relative expression levels of target genes were normalized using Gapdh as an internal control.
Microarray profiling and bioinformatics analysis
Total RNAs from adiponectin treated cells or control cells from three independent experiments were sent to Shanghai OE Biotech Co, Ltd., for microarr ay analysis using an Agilent Whole Human Genome Gene Expression. The slide was scanned using an Agilent scanner, and the output images were digitalized by the Feature Extraction software. The normalization of raw data was then performed using Gene Spring software (version 12.0, Agilent Technologies).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5.0 Software. The significance of differences between groups was assessed by Student’s t test or by analysis of variance (ANOVA) with Student-Newman-Keuls tests for multiple comparisons. A value of P 0.05 (two-tailed) was considered statistically significant.
Results
Adiponectin inhibited the proliferation of human pancreatic cancer cells through its receptors in vitro.
From the study, the data showed that adiponectin treatment significantly inhibited the proliferation of both BxPC-3 cells and CFPAC-1 cells in a dose-dependent manner but had no influence on their migration and invasion in vitro. From the experiment, authors found that exogenous adiponectin treatment caused a more pronounced inhibition of cell growth under low-serum condition in both BxPC-3 and CFPAC-1 cells.
They knocked down the expression of both adiponectin receptors in BxPC-3 cells to evaluate the functional role of AdipoR1/2 via lentivirus-mediated shRNAs targeting AdipoR1 andAdipoR2. The results of the cell proliferation assay showed that knockdown of AdipoR1/2 abolished the antiproliferative effect induced by adiponectin in BxPC-3 cells in the presence of either 10% or 2% FBS in culture medium. These results indicated that adiponectin exerted a significant inhibitory effect on the in vitro proliferation of human pancreatic cancer cells via its receptors and can be further applied in the drug development for pancreatic cancer.
Knockdown of adiponectin receptors promoted the growth of pancreatic cancer in vivo.
Following inoculation of nude mice with BxPC-3-siAdipoR1/2 cells and control cells (BxPC-3-scRNA), respectively. The BxPC-3-siAdipoR1/2 tumors had a drastically faster growth rate than the controls. Compared with the control tumors, the weights of BxPC-3- siAdipoR1/2 tumors were increased by more than 2-fold (0.29-0.06g vs. 0.62-0.13g,) at the time of euthanasia.
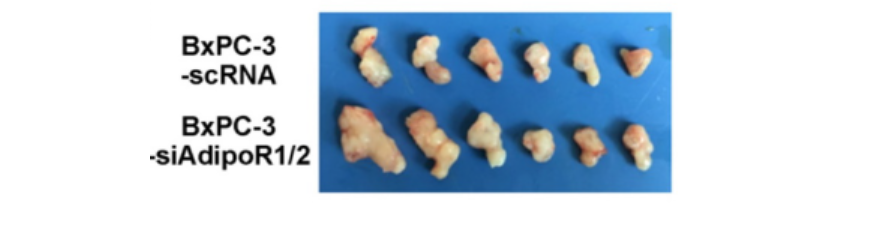
Given the role of adiponectin in inhibiting the proliferation of pancreatic cancer cells in vitro, they detected the expression of PCNA, a maker of cell proliferation, in tumor tissues using immunohistochemistry.
There was a significant increase in PCNA positive cells in BxPC-3-siAdipoR1/2 tumors, demonstrating that blocking adiponectin signaling promoted the proliferation of pancreatic cancer cells in vivo.
Adiponectin induced G1 accumulation in pancreatic cancer cells via suppressing cyclin D1 expression.
The proportion of cells in G0/G1 phase was overtly increased while those in S phase and G2/M phase were reduced, suggesting G1 accumulation in BxPC-3 cells in response to adiponectin treatment. These data indicated that adiponectin suppressed pancreatic cancer cell proliferation through preventing the cells from entering the S phase. Compared to control cells, adiponectin significantly decreased the mRNA expression of cyclin D1 gene (CCND1), but had no effect on other regulators of G1/S transition. Cyclin D1 has been proven to promote the transition from G1 phase to S phase and is mandatory for cell proliferation.
They evaluated the impact of adiponectin in PANC1 pancreatic cancer cells which carries deficient p16. Similar to the results shown in CFPAC-1 and BxPC-3 cells, adiponectin treatment significantly inhibited the cell proliferation and reduced the mRNA level of CCND1. This indicated that the impact of adiponectin on cell growth inhibition and the important role of cyclin D1 in regulating adiponectin-induced G1 accumulation may have a broad implication regardless of whether p16 is defective or not.
Adiponectin down regulated the cellular β-catenin levels via the Akt/GSK3-β pathway in pancreatic cancer cells.
Protein level of β-catenin was evidently decreased after adiponectin treatment. However, there was no change in the β-catenin mRNA expression at the same time point suggesting that adiponectin decrease the β-catenin protein via promoting its degradation. Previous studies have shown that Akt signaling negatively regulates GSK-3β activity by phosphorylating Ser-9 (Jope et al., 2007; Peng et al., 2011), and thus impedes the phosphorylation and degradation of β-catenin. This observation when proven by other researchers, should help drug development to promote degradation of β-catenin and reduce expression of the gene coding for β-catenin mRNA.
These results suggested a suppressive role of adiponectin on regulating the inhibitory phosphorylation of GSK-3β in human pancreatic cancer cells. To confirm that adiponectin promoted the proteasome-mediated degradation of β-catenin via regulation of the GSK-3β pathway, they performed the experiments with GSK-3β inhibitor TWS119 and proteasome inhibitor MG-132. Results showed that although β-catenin in BxPC-3 cells was significantly reduced by adiponectin treatment, such reduction could be completely reversed by TWS119 or MG-132.
BxPC-3 cells were treated with the GSK-3β specific inhibitor TWS119 to further verify the functional role of the GSK-3β signaling pathway in the presence or absence of adiponectin, the cell proliferation assay showed that TWS119 significantly reversed the growth inhibitory effect of adiponectin on BxPC-3 cells. Results suggested that adiponectin promoted the degradation of β-catenin via suppression of the inhibitory phosphorylation of GSK-3β, which contributed to its antiproliferative effect in pancreatic cancer cells.
Adiponectin inhibited the expression of β-catenin-associated transcription factor TCF7L2 in pancreatic cancer cells.
A total of 180 genes were found to be differentially expressed in response to adiponectin treatment, including 102 unregulated genes and 78 down-regulated genes. GO based enrichment analysis was used to classify the adiponectin-induced gene expression changes in pancreatic cancer cells. The results of the analysis for GO cellular component sub ontologies showed that six differentially expressed genes were involved in the transcriptional complex, comprising TCF7L2, TAF5, YAP1, CEBPA, RUNX2 and ANKRD1. Consistent with the microarray results, the results of qPCR revealed the same change in the above six genes. TCF7L2 is a coeffector of β-catenin and can interact with β-catenin to promote target genes activation, including cyclin D1, by recruiting transcriptional complexes (Mao and Byers, 2011). Protein level of TCF7L2 was substantially reduced after adiponectin treatment, suggesting that TCF7L2 may participate in the function of adiponectin.
Critical Analysis of the Results
This study was thoughtfully designed to answer most aspects of the matter under investigation. The findings of the study confirms the adiponectin-induced proliferation inhibitory effect in another two human pancreatic cancer cell lines (BxPC-3 and CFPAC-1). This observation fits with most findings in previous studies (Kelesidis et al., 2006; Illiano, et al., 2017; Salomao et al., 2015). Consistent with their findings, a similar regulatory effect of adiponectin on GSK-3β/β-catenin signaling has also been reported in breast cancer cells (Lam, et al., 2006).
Studies investigating the in vivo effect of adiponectin on murine pancreatic cancer cells reported conflicting results this should form basis for further studies. Their results revealed that adiponectin exerted its inhibitory effect on the growth of pancreatic cancer via modulating the GSK-3β/β-catenin signaling pathway. They found out that adiponectin treatment substantially reduced the serum-induced phosphorylation of GSK-3β, decreased the intracellular accumulation of β-catenin, and down regulated the expression of cyclin D1. These results makes the paper unique, the authors did not just test the hypothesis of the role of adiponectin on pancreatic cancer, through their study, but they dug deeper to unravel the mechanism of the action to molecular level with scientific evidence.

Just like any other scientific research, this paper opens the door to further studies. The debate about PI3K / Akt and Wnt /β-catenin signaling pathways in cancer cells is a field that should be investigated. Akt is possibly involved in the adiponectin induced modulation of GSK-3β/β-catenin signaling. Inhibiting AMPK could alter the phosphorylation level of GSK-3β suggesting AMPK as a molecular link between adiponectin signaling and GSK-3β/β-catenin signaling. Further studies are needed to determine the roles of these two signaling molecules in mediating the adiponectin-induced modulation of GSK-3β/β- catenin signaling in pancreatic cancer cells. In summary, this is a good paper, the study design was well laid out and results of the study have achieved its objective. The only problem with the paper is that abbreviations were used before defining them. The first time one come across abbreviations such as GSK-3β, there is no way to know that it means Glycogen synthase kinase 3 beta until one reach the second last page.
References
- Cheng X, EJ Folco, K Shimizu, (2012). Adiponectin induces pro-inflammatory programs in human macrophages and CD4+ T cells. J Biol Chem; 287(44): 36896-904.
- Davoodi SH, T Malek-Shahabi, A Malekshahi-Moghadam (2013). Obesity as an Important Risk Factor for Certain Types of Cancer. Iranian Journal of Cancer Prevention,; 6(4): 186-194.
- Illiano M, E Nigro, L Sapio, (2017). Adiponectin down-regulates CREB and inhibits proliferation of A549 lung cancer cells. Pulmonary Pharmacology & Therapeutics; 45: 114-120.
- Jope RS, CJ Yuskaitis, E Beurel (2007). Glycogen Synthase Kinase-3 (GSK3): Inflammation, Diseases, and Therapeutics. Neurochemical research; 32(4-5): 577-595.
- Kelesidis I, T Kelesidis, and CS Mantzoros(2006). Adiponectin and cancer: a systematic review. Br J Cancer; 94(9): 1221-5.
- Kern PA, GB Di Gregorio, T Lu, (2003). Adiponectin Expression From Human Adipose Tissue. Diabetes; 52(7): 1779.
- Lauby-Secretan B, C Scoccianti, D Loomis, (2016). Body Fatness and Cancer — Viewpoint of the IARC Working Group. New England Journal of Medicine; 375(8): 794-798.
- Ligibel JA, CM Alfano, KS Courneya (2014). American Society of Clinical Oncology Position Statement on Obesity and Cancer. Journal of Clinical Oncology; 32(31): 3568-3574.
- Mao CD, SW Byers (2011). Cell-Context Dependent TCF/LEF Expression and Function: Alternative Tales of Repression, De-Repression and Activation Potentials. Critical reviews in eukaryotic gene expression; 21(3): 207-236.
- Nam SY. Obesity-Related Digestive Diseases and Their Pathophysiology. Gut and Liver, 2017; 11(3): 323-334.
- Siegel AB, A Goyal, M Salomao,(2015). Serum Adiponectin is Associated with Worsened Overall Survival in a Prospective Cohort of Hepatocellular Carcinoma Patients. Oncology, ; 88(1): 57-68.
- Siegel RL, KD Miller, A Jemal (2016) Cancer statistics. CA Cancer J Clin, 2016; 66(1): 7-30.
- Torre LA, F Bray,(2012) RL Siegel Global cancer statistics,. CA Cancer J Clin, 2015; 65(2): 87-108.
- Wang C-Y, A-C Tsai, C-Y Peng, (2012). Dehydrocostuslactone Suppresses Angiogenesis In Vitro and In Vivo through Inhibition of Akt/GSK-3β and mTOR Signaling Pathways. PLoS ONE, 2012; 7(2): e31195.
- Wang Y, JB Lam, KS Lam,( 2006). Adiponectin modulates the glycogen synthase kinase 3 beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res,; 66(23): 11462-70.
- Yamauchi T, J Kamon, Y Minokoshi, (2002). Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med; 8(11): 1288-95.
Continue your exploration of Acoustic Wave On Soft Matter with our related content.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



