NCI's COMPARE Algorithm: Revolutionizing Anti-Cancer Screening
DISCUSSION
A huge amount of data is generated by the Developmental Therapeutics Programs (DTPanti-cancer) screening program and is maintained in a computerized database. Data analysis dissertation help is one of the most essential sources for researchers to navigate through this vast sea of information. A compare analysis is a technique for comparing items and evaluating their similarities and differences (1). The COMPARE algorithm has considerably boosted the utility of the live persons tumor cell line assay as a discovery tool. The most recent in vitro-screen, which is used to evaluate the performance of synthetic and organic compounds, was developed over several years and launched in April 1990. A total of 59 tumor tissue cells from humans have been divided into disease subpanels. Each month, the screen evaluates about 1,000 molecules and natural substance extracts at maximum capacity (3).The decision to not change the assay technique or cell lines used in the screen for an extended time allowed vast numbers of molecules to be evaluated under similar circumstances. COMPARE analyzes the resulting data. The NCI accession number of a probe or "seed" compound is used to identify the compound a so-called (NSC number).

COMPARE ranks the entire archive of tested components in terms of how similar their cytotoxicity is to that of other compounds in the database (1). The Pearson correlation coefficient indicates how closely the pattern resembles that of the seed. According to the COMPARE algorithm, compounds near the top of the list may have a mechanism of action similar to the seed compound. It was proposed in 1985 that a screen could find cell-type-specific agents with clinical activity against solid tumors by looking for them (6). The realization that in vitro histology has a poor correlation with patient care is beginning to emerge, but the sequence of cell membrane responsiveness and drug reaction in cell lines correlates with molecular goal affirmation.
The National Cancer Institute's (NCI) cytotoxicity screen, which has been in operation since 1990 as part of the Developmental Therapeutics Program (DTP), provides for the free examination of compounds with potential anti-cancer action (2). The first stage involves a single 10M treatment to construct a mean growth percent graph for the 60 human tumor cell lines derived from nine key histological tissue types: breast, colon, central nervous system, leukemia, melanoma, non-small cell lung, prostate, ovary, and renal cancers. The NCI then chooses compounds for stage 2 evaluation, which includes five dose screening, allowing GI50, LC50, and TGI to be determined.
- The GI50 value is the amount of a substance necessary to inhibit cell growth by 50%.
- The LC50 value is the concentration of a substance that is necessary to reduce the cell population by 50%.
- TGI is the amount of a substance that is necessary to have a cytostatic effect.
Take a deeper dive into Muslim Female Therapists with our additional resources.
Continue your exploration of Muslim Female Therapists with our related content.
The specified parameters serve as the seed vector for the NCI COMPARE algorithm, which generates cytotoxicity profiles that are almost identical. COMPARE allows you to make comparisons against a database of standard agents (3). The standard agents database contains over 200 well-documented anti-tumour drugs with hypothesized mechanisms of action. COMPARE also permits comparisons to be made using the NCI database's 70000 synthetic chemicals.
The degree of similarity or lack thereof between two cytotoxicity profiles is determined using a commercially available SAS statistical tool to calculate a Pearson product moment correlation coefficient (0- 1) to identify the degree of similarity or lack thereof. 16,17 Pearsons in the (0.3-0.5) range are regarded medium, and those above (0.5) are called strong (7). 18 Negative correlation coefficients between the test and correlated substance show a broad inverse association for the seed parameter across the 60 human tumor cell lines. The COMPARE program enables the determination of a test substance's possible mechanism of action if its cytotoxicity response shares strong similarities with a compound whose mode of action is known.
Alternatively, if a biological reaction correlates to the activity of a known molecular target, the method of action can be determined. Following are some instances of how the COMPARE program has been used using COMPARE (6). Paull et al. developed a variety of novel antimitotic drugs that operate by inhibiting tubulin polymerisation and triggering mitotic arrest in cultured cells 82 compounds were discovered using COMPARE correlation using the cytotoxicity response profiles of ten recognized antimitotic drugs, including taxol, vincristine, and vinblastine, as COMPARE seeds( 8). Fifty compounds were identified as seeds or seed analogues, 32 as novel compounds, and 19 as separate chemical species having a correlation coefficient of 0.6 or more to the seed compounds and a GI50 of 1M or less towards the HL-60 human leukemia cell-line. In vitro tubulin polymerisation was inhibited by 23 of the 32 compounds, with the majority identified by six or more seeds.
Continue your journey with our comprehensive guide to Societal Impact in a Comparative Analysis.
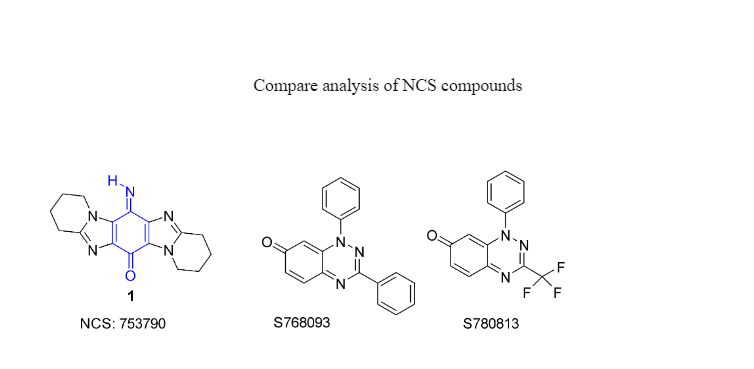
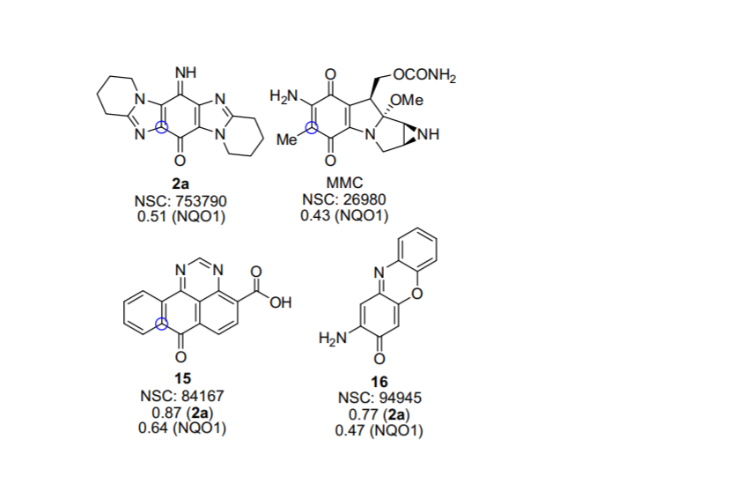
Fagan et al. recently synthesized iminoquinone (NSC 753790), which had a higher correlation of 0.51 than mitomycin C (MMC), which had a correlation of 0.43 towards NAD(P)H:quinone oxidoreductase 1. (NQO1). This enzyme, which is overexpressed in many human tumor cell lines, has a known substrate in MMC. The cytotoxic effect of NQO1 reductive activation of prodrugs (12). Computational docking confirmed the iminoquinone and MMC as NQO1 active site substrates. COMPARE analysis recently revealed an alternative mode of action for a series of iridium complexes in comparison to cisplatin and related transition metal complexes, and sesamin, a compound with known anticancer activity, was subjected to standard and reverse COMPARE analysis to identify genes responsible for resistance and sensitivity to sesamin (6).
Standard COMPARE associates a chemical with high levels of molecular target, whereas reverse COMPARE associates a compound with low levels of molecular target or where a molecular target confers resistance. Kunkel et al. used the cytotoxicity profiles of known thioredoxin reductase inhibitors, 1-methylpropyl 2-imizolyl-disulfide (IV-2) and benzyl-2-mercaptoimidazolyl disulphide (DLK-36), as COMPARE seeds to identify novel thioredoxin reductase inhibitors (7). Pleurotin NSC 401005 exhibited the highest correlation (0.836) to IV-2 from the synthetic chemical database, while pleurotin and NSC 665103 were the most powerful inhibitors in a thioredoxin reductase/thioredoxin-dependent insulin reductase experiment. Furthermore, when submitted to a thioredoxin reductase/thioredoxin assay, 77 percent of the compounds found from the NCI database had IC50 values of 10 g/ml or less, while 32 percent had IC50 values of 1 g/ml (12).
The 1,3-diphenylbenzo[1,2,4]triazin-7-ones 1a-1l are known as NSC 768093, and they are formed from 1-phenyl-3- (trifluoro-methyl)benzo[1,2,4]triazin-7-one (NSC 780813) and 1,3-diphenylbenzo[1,2,4]triazin-4-yl radical. One dose (10M) of 1,3-diphenylbenzo[1,2,4]triazin-7-ones 1a-1l exhibits substantial inhibition of development in many cancer cells, including colon, leukaemia, renal, and melanoma malignancies, according to NCI (). Out of 70,000 chemicals in the NCI database, pleurotin had the greatest correlation co-efficient to benzothiazine 1a, with a Pearsoncoefficient of 0.84, indicating a strong association.
Take a deeper dive into Analysis of New Media Productions with our additional resources.

The compounds' growth inhibition (GI50), total growth inhibition (TGI), and lethal concentration (LC50) characteristics were analyzed using the NCI screening method, and the half-maximal inhibitory concentration (IC50) of these molecules was determined using the Four-Parameter Logistic Function as well. When the TGI and IC50 values of the compounds were evaluated, it was discovered that the tested compounds had selective antitumor activities against all cell lines tested. Although compound 2 has an antiproliferative effect (IC50 values ranging from 4.5 to 20.7 g/mL; TGI values ranging from 4.4 to 20.9 g/mL) against FL, HeLa, and Hep3B cell lines (Table 1 and 2), compound 4 has strong antitumoral properties (IC50 values ranging from 7.8 to 38.9 g/mL; TGI values ranging from 7.9 to 40.1 g/mL) against FL.
Compound 1 has a considerable antiproliferative effect on FL cells (IC50 value 1.1 and TGI value 1.1 g/mL) (Table 1). Compounds 1 (IC50 value 10.4 and TGI value 10.6 g/mL) and 2 (IC50 value 9.5 and TGI value 9.6 g/mL) demonstrated substantial anticancer activity in Hep3B cells (Table 2). When the IC50 and TGI values of all of the above-mentioned chemicals are taken into account, the effective ones have better antiproliferative effects than the positive control group, cisplatin and 5-FU (Tables 1 and 2). Furthermore, when the low GI50 values (1 - 2 g/mL) and high LC50 values (40 - 400 g/mL) are evaluated, the active compounds can be exploited in advanced pharmacological studies (Table 1 and 2).
It is critical for a substance's harmful effect on normal cells to be as low as possible. As a result, the anticancer and cytotoxic activities of these compounds should be compared in order to determine each compound's forward pharmacological potential. The LDH cytotoxicity assay was used to examine the cytotoxicity of the substances in cells, indicating membrane damage indirectly. When cytoplasmic LDH activity measurement results for these compounds are analyzed, it is discovered that compounds 1-4 for A549 and Hep3B cell lines, compounds 1 and 4 for MCF7 and HeLa cell lines, compounds 1 and 3 for HT29 cell lines, and compounds 2 and 4 for FL cell lines cause approximately 7% to 27% membrane damage at their IC50 concentration.
The Minimum Inhibition Concentration (MIC) approach was used to analyze the results. At 250 g/mL and below the MIC values, we regarded our test compounds to be antibacterial. The compounds' MIC values were compared to the values of antibacterial medicines employed as positive controls. When the MIC values of recently prepared molecules were tested on Gram (+) bacteria, the antibacterial effect of compounds 1 and 2 against E. faecalis (VRE) ATCC 19433 (62.5 – 250 g/mL), compound 1 against E. faecalis ATCC 29212 (250 g/mL), compounds 1-3 against S. aureus ATCC 25923 (31.25 – 62.50 g/mL), compounds 1, 2 and 4 against
To the cancer cell lines tested, benzotriazin-4-yl radicals were significantly less cytotoxic than their oxidation products, benzo[1,2,4]triazin-7-ones. Using the MTT assay, pyridyl-substituted benzotriazin-7-ones had submicromolar cytotoxicity comparable to 1,3-bisphenylbenzo[1,2,4]triazin-7-one 1 (4). Because of the variable DTP-NCI one-dose testing cytotoxicity profiles, they were chosen for five-dose testing. Despite the overall greater cytotoxicity of the pyrid-2-yl-substituted compounds compared to 1after one-dose testing, COMPARE analysis revealed very strong correlations to pleurotin (2). Complete one- and five-dose data for can be found in the Supplementary Information accompanying this research. The NCI sampling for subsequent three-dose screening formed significant components used in the NCI COMPARE algorithm to determine closely matching cytotoxicity profiles. The COMPARE analysis made cytotoxicity comparisons with the NCI's vast database of over 250,000 synthetic compounds much easier. The Pearson product-moment correlation coefficient (0 to 1) describes the degree of similarity between two cytotoxicity profiles, with values above 0.5 considered strong (12). The Pearson correlation coefficient of 1 is comparable.
With cytotoxicity profiles similar to the irreversible TrxR inhibitor pleurotin, the 1,3-substitution in the benzo[1,2,4]triazin-7-one scaffold from Ph to pyrid-2-yl did not appear to change the compound's mechanism of action. The cytotoxicity of the 3-substituted 1-phenylbenzo[1,2,4]triazin-7-ones had a very strong correlation (Pearson correlation coefficients of 0.8) to the naturally occurring antibiotic and anti-cancer agent pleurotin, according to the National Cancer Institute (NCI) COMPARE analysis (12). The anti-cancer activity is thought to be due to thioredoxin reductase (TrxR) inhibition, with the 3-Ph substituted benzotriazinones showing strong reversible mixed and uncompetitive inhibition. Evidence that scaffold analogues were multi-target inhibitors of Alzheimer's disease prompted our anti-cancer research (2). At room temperature, Blatter-type (benzotriazin-4-yl) radicals are treated with manganese dioxide in dichloromethane to produce benzotriazinones.
The survival of benzo[1,2,4]triazin-7-ones and 1,2,4-benzotriazinyl (Blatter-type) radical precursors in cells is characterized, with correlations to 2,2,6,6-tetramethyl-1-piperidinyloxy radical precursors (TEMPO) (2). The benzo[1,2,4]triazin-7-ones were several orders of magnitude less cytotoxic than the steady free - radical. The formulation and examination of two new pyrid-2-yl benzo[1,2,4]triazin-7-ones are defined, with the 1,3-substitution from phenyl to pyrid-2-yl increasing cytotoxic effects against most cancer cell lines, as evidenced by one-dose testing at the National Cancer Institute. The NCI's three-dose testing data showed very strong correlations to the naturally occurring anti-cancer compound pleurotin, according to a COMPARE analysis (9). COMPARE is a program that compares cytotoxicity data from different compounds and allows for quantitative expression as Pearson correlation coefficients. Compounds were also assessed using an independent MTT assay, which was compared to data generated by the SRB assay data.
Recently synthesized disubstituted tacrine with six or seven membered hydrocycles were investigated in vitro for antibacterial and anticancer activity. We demonstrated that disubstituted tacrines have significant potential as anticancer and antibacterial agents. Depending on the replacement group on the tacrine ring, the test results describe both a good antiproliferative impact and a modest cytotoxic effect. In vitro experiments have shown that the mono silyl substituted seven membered tacrine analogue 3 binds the DNA of cancer cells. According to our findings, four disubstituted tacrine derivatives are interesting anticancer and antibacterial therapeutic candidates, however more pharmacological testing is needed.

Reference
Apasu JE, Schuette D, LaRanger R, Steinle JA, Nguyen LD, Grosshans HK, Zhang M, Cai WL, Yan Q, Robert ME et al (2019) Neuronal calcium sensor 1 (NCS1) promotes motility and metastatic spread of breast cancer cells in vitro and in vivo . FASEB J 33, 4802–4813. [PMC free article] [PubMed] [Google Scholar]
Azimi I, Milevskiy M, Chalmers S, Yapa K, Robitaille M, Henry C, Baillie G, Thompson E, Roberts‐Thomson S and Monteith G (2019). ORAI1 and ORAI3 in breast cancer molecular subtypes and the identification of ORAI3 as a hypoxia sensitive gene and a regulator of hypoxia responses. Cancers 11, 208. [PMC free article] [PubMed] [Google Scholar]
Bassett JJ, Bong AHL, Janke EK, Robitaille M, Roberts‐Thomson SJ, Peters AA and Monteith GR (2018) Assessment of cytosolic free calcium changes during ceramide‐induced cell death in MDA‐MB‐231 breast cancer cells expressing the calcium sensor GCaMP6m. Cell Calcium 72, 39–50. [PubMed] [Google Scholar]
Berridge MJ (2016) The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 96, 1261–1296. [PubMed] [Google Scholar]
Bittremieux M, Parys JB, Pinton P and Bultynck G (2016) ER functions of oncogenes and tumor suppressors: modulators of intracellular Ca2+ signaling. Biochim Biophys Acta 1863, 1364–1378. [PubMed] [Google Scholar]
Boeckel GR and Ehrlich BE (2018) NCS‐1 is a regulator of calcium signaling in health and disease. Biochim Biophys Acta 1865, 1660–1667. [PMC free article] [PubMed] [Google Scholar]
dtp.cancer.gov 2021, Developmental Therapeutics Program, Available at: https://dtp.cancer.gov/ [Accessed on: 27 November 2021]
dtp.cancer.gov 2021a, Screening Procedures, Available at https://dtp.cancer.gov/databases_tools/docs/compare/compare_methodology.htm [Accessed on: 27 November 2021]
dtp.cancer.gov 2021b, COMPARE Analysis, Available at: https://dtp.cancer.gov/databases_tools/compare.htm [Accessed on: 27 November 2021]
Fagan, V., Bonham, S., Carty, M.P., Saenz-Méndez, P., Eriksson, L.A. and Aldabbagh, F., 2016. COMPARE analysis of the toxicity of an iminoquinone derivative of the imidazo [5, 4-f] benzimidazoles with NAD (P) H: quinone oxidoreductase 1 (NQO1) activity and computational docking of quinones as NQO1 substrates. Bioorganic & medicinal chemistry, 20(10), pp.3223-3232.
Humphreys, R.K., Puth, M.T., Neuhäuser, M. and Ruxton, G.D., 2019. Underestimation of Pearson's product-moment correlation statistic. Oecologia, 189(1), pp.1-7.
Obilor, E.I. and Amadi, E.C., 2018. Test for significance of Pearson’s correlation coefficient. International Journal of Innovative Mathematics, Statistics & Energy Policies, 6(1), pp.11-23.
Continue your exploration of Police Corruption in Zambia as a form of Institutional Corruption with our related content.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



