Understanding Waxy Crude Oils
INTRODUCTION
Waxy crude oils have been produced for several decades. The amount of wax present in the crude oil is less than 5%, but even such a small percentage can cause complications for the upstream industry. Deposition of wax crystals brings serious operational and economic challenges.1 Wax precipitates from the crude oil when the oil cools below the wax appearance temperature (WAT) and a temperature gradient exist between the oil and the colder deposition surface. There are several methods of preventing or removing sediments. One of the preventive techniques is the addition of chemicals, known as wax inhibitors.2 Wax inhibitors are usually polymeric structures containing polar and non-polar moieties. The non-polar moiety interacts with waxes whereas the polar group disrupts wax crystal growth, regulate morphology, and inhibits the formation of large crystals. There are some polymeric structures used as flow improvers. The most common are ethylene-vinyl acetate (EVA) and poly(ethylene-butene) (PEB). Ethylene-vinyl acetate 30 is the most efficient copolymer for pour point depression, although the presence of petroleum asphalt residue reduces the efficiency of the EVA copolymers and are more efficient for systems containing high carbon number paraffin.3 Despite the success of these materials, there still exist areas for continued research in the field of new inhibitors. In 2016, Xue Yan described a “star-like” material based on β-cyclodextrin for enhancing the flowability of paraffinic oils obtained by the catalytic esterification of β-cyclodextrin with palmitic acid under mild conditions.4 The author subsequently described the behavior of these new a “star-like” structures in a waxy oil concluding in the significant reduction of wax appearance temperature (WAT).13 Based on this idea a new “star-like” structure is synthesized by esterification of the hydroxyl groups on the exterior shell of β-cyclodextrin with carboxylic acids. The report will divide into two main sections. In the first, the synthesis of the inhibitors and structural characterization using IR and 1H-NMR spectroscopies will be described, and in the second part the main focus will be on wax appearance temperature (WAT) measured by differential scanning calorimetry (DSC) and the morphology of the crystals which were observed under cross-polarized microscopy (CPM).
BACKGROUND
β-CYCLODEXTRIN
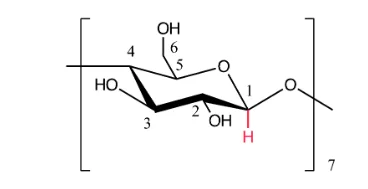
β-cyclodextrin is a cyclic oligosaccharide composed of seven glucose units. Each unit consists of three hydroxyl groups; the primary hydroxyl group is in the first position, while secondary hydroxyl groups occupy the second and third positions.5 The shape of the molecule is a cone-like shape with a strong intramolecular hydrogen bonding between the hydroxyl groups at the position 2 and 3. The exterior of the molecule is hydrophilic whereas the inner part of the molecule is rather lipophilic. The cavity of β-cyclodextrin is large enough to hold up some molecules, which makes them very attractive in terms of formation inclusion complexes.6 Thus, cyclodextrins are widely used in the pharmaceutical industry and food processing.

STEGLICH ESTERIFICATION MECHANISM
In 1978 Wolfgang Steglich described a new esterification method, known as Steglich esterification.7 The reaction was developed to esterify sterically hindered alcohols with DCC as a coupling reagent and DMAP as a catalyst. Figure 2.1.1 represents the general reaction for the β-cyclodextrin and myristic acid.
Looking for further insights on Acidity in Crude Oil? Click here.

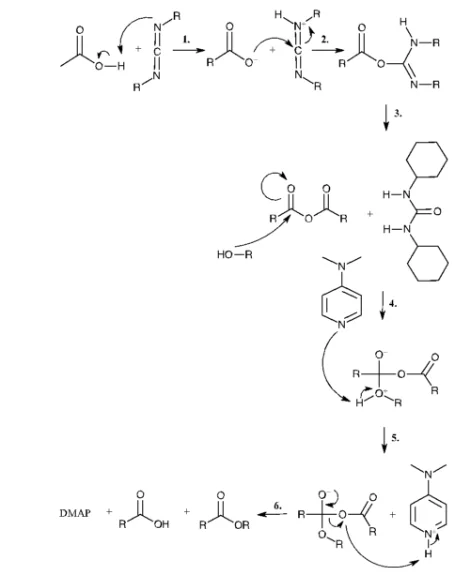
The first step is the reaction of a carboxylic acid with DCC to produce O-acylisourea, which is more reactive than the carboxylic acid used in the synthesis (Figure 2.2.2).
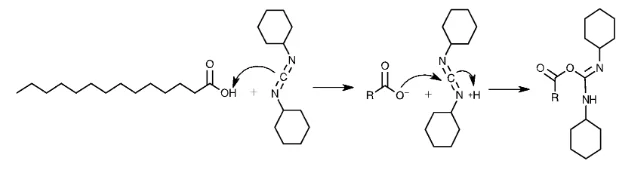
Subsequently, the alcohol reacts with the intermediate to form dicyclohexylurea and the corresponding ester. If the rate of the reaction is slow, a side reaction occurs resulting in a reduced yield. Addition of DMAP suppresses the formation of the side products.11 The mechanism of DMAP and the O-acylisourea forming the intermediate which will rapidly react with β-cyclodextrin is schematically represented in Figure 2.2.3.


3. MATERIALS AND METHODS
3.1 MATERIAL
3.1.1. Materials for synthesis:
Chemicals used throughout the experiment were commercially available from SIGMA-ALDRICH (Merck, Darmstadt, Germany). Myristic acid (99%), stearic acid for synthesis, N,N’-dicyclohexylcarbodiimide (DCC) (99%), 4-(dimethylamino) pyridine (DMAP) (99%), dimethylformamide anhydrous (DMF) (99.8%), heptane (99%), hydrochloric acid (HCl) (32%), sodium chloride (NaCl) (99%) , sodium bicarbonate (NaHCO3) (99.5%), β-cyclodextrin (98%) , paraffin wax, chloroform (CHCl3) (99.9 %), D2O (99.9 %).
3.2. METHODS
3.2.1. STEGLICH ESTERIFICATION METHOD
Esterification of β-cyclodextrin was carried out in the pre-dissolved mixture of dry DMF (12 ml), myristic acid (1.809 g) and N,N’-Dicyclohexylcarbodiimide (1.2677 g), which was magnetically stirred for 30 minutes in an ice bath. After, DMAP (0.09 g) was added into the reaction mixture, followed by the slow addition (dropwise) of the pre-dissolved β-cyclodextrin (1.002 g) in DMF (20 ml). The reaction was kept in an ice bath for 90 minutes and then slowly raised to room temperature. The reaction was run under argon conditions for 27 hours and stirred, simultaneously. The product was filtrated and washed with 60 ml of HCl (0.1 M), NaHCO3 (7.5 wt%), NaCl (10 wt%) and water (500 ml), respectively. Subsequently, it was left for drying in the vacuum oven at 70 0C for 48 hours, yield (1.35 g). The same method was used for the preparation of stearic-cyclodextrin (S-CD) and a mixture of two acids; stearic acid and myristic acid (MS-CD). Although, the masses used in the reaction for S-CD and MS-CD mixture of two are different as everything was recalculated due to different molar masses of acids (appendix 4).
3.2.2. MODEL OIL AND SAMPLE PREPARATION
15 wt% (w/w) oil was prepared by dissolving n-paraffin (12.002 g) in the heptane (100 ml), followed by heating and stirring at 45 0C on a hot plate until the homogenous, clear mixture was observed. Likewise, 26 wt% (w/w) oil was prepared by dissolving n-paraffin (24.000 g) in the heptane (100 ml) stirred and heated on a hot plate until homogeneous it. 1, 3, 5 and 10 w% of M-CD inhibitor was added to the model oils (15 wt%, 26 wt%) and the real oil. The mixture was heated in the preheated oven (50 0 C) for 5-10 minutes to enable the wax inhibitor to dissolve. The same procedure was repeated for S-CD and MS-CD inhibitors. These mixtures were used in all subsequent measurements.
CHARACTERIZATION METHOD AND EXPERIMENTAL PROCEDURE
The chemical structures of the products were characterized by Perkin Elmer FT-IR and 1H-NMR (400 MHz, Bruker) spectroscopies. Firstly, β-cyclodextrin (6.23 mg) was dissolved in D2O (5 ml) while myristic acid (4.23 mg) and the product M-CD (6.09 mg) were prepared in the CDCl3. The analyses were performed at room temperature, and the obtained spectra were used for further characterization of the product. Differential Scanning Calorimetry (DSC 821 – METTLER TOLEDO) was used to determine the wax appearance temperature (WAT) for the model oils (15 wt %, 26 wt %) and the real oil, with and without the inhibitors. Each sample (~20 mg) was preheated in the oven (50 0C) and transferred by Pasteur transfer pipette into the pre-weighed aluminum pan, sealed and accurately weighted. The experiment was performed on cooling program 70(-40) 0C at a rate of 10 0C per minute. The first appearance of the wax crystals and the morphology of the crystals present in the mixtures were observed through cross-polarized microscopy (CPM). The sample was transferred onto a glass slide and heated up to 70 0C. Once the sample was preheated, it was cooled down at a rate of 5 0C per minute until the first crystals were observed. Thermal gravimetric analyses (TGA) were performed using a TA Instruments Q500 (Perkin Elmer).
RESULTS AND DISCUSSION
The synthetic approach was based on what was described by Xue Yan, 2016 but with some modifications. First, the solvent used for the synthesis was dimethyl furan. When β-cyclodextrin was added to dimethylfuran, however, a heterogeneous mixture was observed. Attempts to dissolve the compound by sonication and increasing temperature were not successful. Thus, the choice of the solvent for all following syntheses was N, N-dimethylformamide anhydrous. As mentioned previously, the Steglich esterification is a sterically demanding reaction which has to be run in mild conditions for 27 hours. The first syntheses which were performed were not successful due to the production of a by-product and a low ester yield. To increase the number of esterified sites on the β-cyclodextrin, the ratio of acid:β-cyclodextrin of 1 to 6:33 was increased to 1 to 7:33 resulting in a higher number of esterified sites. All three inhibitors described in this report were synthesized using the higher ratio.
CHARACTERIZATION OF THE NEWLY SYNTHESIZED COMPOUNDS
4.1.1. Myristic-β-cyclodextrin (M-CD)
The initial characterization of M-CD was performed by FT-IR spectroscopy. The IR spectrum for the myristic acid was very similar to that of M-CD (Figure 4.1a). Peaks present in the region of 2963-2871 cm-1 correspond to sp3 stretches which are present in both spectra. IR spectrum for myristic acid contains a peak at 1695 cm-1 corresponding to a carbonyl group in an acid which diminishes in the M-CD IR spectrum. Instead, the M-CD IR spectrum displays the peak in the region of 1740 cm-1 which corresponds to the carbonyl stretch belonging to the ester group suggesting the linkage between ß-cyclodextrin and myristic acid.

Figure 4.1b shows a 1H-NMR spectrum of M-CD compound which was used to determine the esterification ratio of β-cyclodextrin and myristic acid. The relative value was calculated from the integration of hydrogen in the position one which is shown in Figure 4.1c. (1H-NMR 4.1 ppm). In the 1H-NMR spectrum, the most shielded peak corresponds to -CH3 group (1H-NMR, 0.9 ppm) integrating for 16.2 protons. Knowing the number of protons represented by these integrals, it could be calculated that there are about 5.4 alkyl chains attached to β-cyclodextrin molecule, suggesting that each ß-cyclodextrin molecule has approximately five alkyl chains attached on it. Additionally, two singlets just below 3 ppm and singlet peak at 8 ppm correspond to DMF solvent which was used as a solvent throughout the experiment.
Stearic-β-cyclodextrin (S-CD) characterisation
Esterification of the hydroxyl groups on the exterior shell of β-cyclodextrin with stearic acid was performed several times as it was difficult to obtain reproducible data. Eventually, after leaving the reaction for 48 hours rather than 27 hours we obtained more pronounced ester peak in the region of 1740 cm-1. The FT-IR spectrum of S-CD is shown in Figure 4.2a. It can be seen that stearic acid shows C=O peak at 1698 cm-1 corresponding to the carboxyl group and C-H stretches just about 3000 cm-1. The newly synthesized compound, S-CD shows no peak belonging to the carboxyl group, but in the carbonyl region, some changes were observed.

FT-IR spectrum of S-CD shows the band at 1741 cm-1 which corresponds to ester group and an anhydride peak in the region of 1800 cm-1. Generally, anhydrides are very unstable in the aqueous solutions and therefore there was no clear explanation for an anhydride being in the mixture of our final product. The assumption was made, and the presence of an anhydride could be explained by the following argument. The reaction of stearic acid and DCC produced O-acylisourea, which reacted with the additional stearic acid and formed an acid anhydride. Due to a unique structure of β-cyclodextrin and its amphipathic properties, the anhydride could be encapsulated in the interior of the molecule. The proposed mechanism is represented in Figure 4.2.2.
In the 1H-NMR, a signal integrating for seven hydrogens was found at 4.07 ppm (Figure 4.2b). The calculated esterification ratio for S-CD is 6.9 suggesting that each β-cyclodextrin molecule contains approximately seven alkyl chains within the molecule. It is noted, however, that the product does contain both anhydride and ester and thus the number of chains per ß-cyclodextrin molecule is uncertain.
Myristic-Stearic-β-cyclodextrin (MS-CD) characterisation
MS-CD was prepared from equimolar quantities of myristic and stearic acid. Figure 4.1.3a shows FT-IR spectrum for MS-CD compound. As shown, an absorption band in the region of 1741 cm-1 corresponds to carbonyl stretch belonging to the ester group, and the stretching vibration in the region of 1803 cm-1 corresponds to an anhydride group. We tried to purify one of the products by dissolving 0.5 g MS-CD in DCM (30 ml). The flask was sealed and left in the freezer for 3 hours. After, the product was filtered over celite/silica gel and washed with DCM. Afterward, the solvent was removed from the solution by using the rotary evaporator, dried and analyzed. The peak in the region of 1800 cm-1 still presents the FT-IR spectrum. Based on this discretion, the purification of other products wasn’t performed.

Figure 4.1.3b shows a 1H-NMR spectrum of MS-CD inhibitor. The signals in the spectrum are overlapping in a greater extent than signals in M-CD or S-CD 1H-NMR spectra what is due to different chain length attached on the ß-cyclodextrin molecule. However, we were able to identify hydrogen 1 (Figure 4.1c) integrating for seven protons (4.3 ppm). Calculated ratio for MS-CD is 6.0, suggesting six alkyl chains being attached on the ß-cyclodextrin molecule.
PHYSICAL PROPERTIES OF THE NEW INHIBITORS
The physical properties of the newly synthesized inhibitors are present in table 4.2. The thermal stability of the inhibitors significantly varies if we take into the consideration the fact that these three inhibitors were synthesized by the same method and only difference is the length of the carboxylic acids being attached on the ß-cyclodextrin molecule. Decomposition of the ß-cyclodextrin shows two regions. The first region below 200 0C is associated with the elimination of hydroxyl groups and the second step is decomposition of the ß-cyclodextrin residue.8 As can be seen, S-CD loss 5 % weight, below 200 0C which might be a good indication that the S-CD hasn’t been extensively esterified as it was predicted from the calculated value from the 1H-NMR. The highest stability is observed in the M-CD with a weight loss of 5% at 258 0C. Degradation pathway for all three inhibitors is slightly odd because for three identical compounds; essentially, it would be expected that when heated, the decomposition mechanism would be similar. That is pointing that these materials are not similar as it was predicted in the first place (appendix).
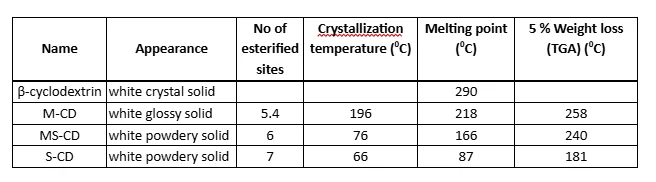
The melting point for the ß-cyclodextrin molecule is between 290 and 300 0C.10 High melting temperature for such a large molecule is associated with the intramolecular hydrogen bonding. Substitution of hydroxyl groups with long alkyl chains causes destabilization of the molecule and therefore lower the melting point of the compound. As it is shown in table 4.2., the melting point decreases with increasing number of disruption in the molecule and also affects the crystallinity of the compounds. As it is shown in figure 4.2.1, M-CD shows crystalline sites which could be associated with the regular chain length in the molecule.

WAX APPEARANCE TEMPERATURES
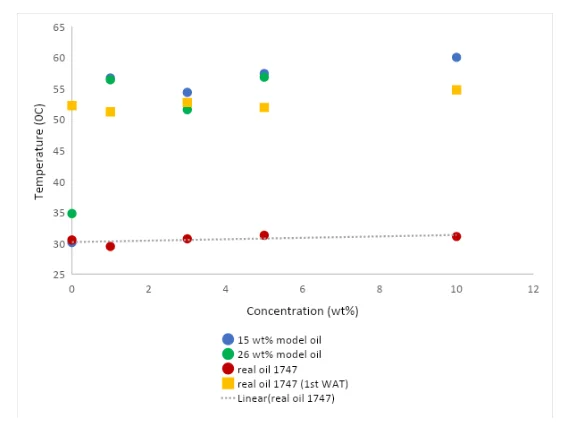
Differential scanning calorimetry and cross-polarized microscopy were used as the complementary techniques to study wax crystallization of the model and real oils. In DSC, separation of the solid phase from the homogenous mixture appeared at 34.8 0C in 26 wt% model oil, and for the 15 wt% model oil, the WAT was 30.1 0C. DSC measurements showed two distinct regions for WAT in the real oil. The first crystal appearance was recorded at 52.2 0C and the second fraction appeared at 30.2 0C. From the application point of view, the high-temperature fraction is important because the formation of the crystals at that temperature might well seed the formation of additional crystals. The WAT and the morphology of the wax crystals were observed by CPM technique. Figure 4.3 represents the wax crystals in three different types of oils, which were taken at the room temperature.

In the crystallization processes, temperature is one of the main factors affecting the wax crystal precipitation and its growth. As the temperature decreases so do the kinetic energy of the wax, therefore the motion of the paraffin molecules is restricted, causing the reduction of the free space between the molecules. When the molecules get closer together, they tangle and form clusters which grow and become stable. In the oil systems, nucleation and crystal growth develop simultaneously.9 It is noted that the morphology of the wax crystals is highly dependent on the percentage of the wax and type of paraffin present in the oil. It can be observed that 26 wt% waxy oil has large, well-defined crystals with a needle-like shape. In 15 wt% waxy oil the crystals are smaller, but the amount of the crystals is higher figure 4.3. The composition of real oil is very different from that of the model oil and as could be expected the size of the crystals is very small compared to the size of the 26 wt%. That could be explained by the fact that the composition of crude oil is rich in different compounds which influence the size and shape of the wax crystals. Figure 4.3.1 represents the plots of the WAT versus concentration of M-CD which was added into three different types of oils. By analyzing this graph, it could be observed that WAT increased by 18.3 0C in 15 wt% oil by adding 1 % of M-CD, and in 26 wt% model oil the wax appearance temperature appeared to increase by 21.2 0C. The highest increased in WAT in all three oils was observed by adding 3 % of M-CD. After adding the higher amount of M-CD, the WAT stayed constant with slight deviation ± 0.9 0C. From figure 4.3.1. it can also be observed that addition of M-CD has no significant effect on WAT in the real oil samples. Moreover, by adding 1 % M-CD into the real oil sample there was decrease in WAT by 1.2 0C what is the opposite effect what could be observed in the model oils where temperature significantly increased by addition of 1 % M-CD.


Figure 4.3.2 shows a wax precipitation process of the model and real oils. The first appearance of the wax crystals is in very good correlation with WAT (±2 0C) which was measured by DSC. The reduction of temperature in the constant rate produced the higher number of the wax crystals, and the crystal growth, simultaneously. It is noted however that the morphology of the crystals in the model oil differs to that of the real oil. The shape of the crystals in the model oil is a needle-like shape whereas in the real oil is more like spherulites. The morphology of the crystals is not only influenced by the composition of the oil but also by the type and concentration of the inhibitor added into the oil.

Figure 4.3.3 and Figure 4.3.4 show the relationship between WAT and inhibitor concentration added into the oils. The behavior of the S-CD and MS-CD inhibitors in the waxy oils was very similar to that of M-CD inhibitor. In both cases, S-CD and MS-CD, had a minimal effect on WAT in the real oil samples, and a significant increase in WAT in the model oils. WAT in the real oil was between 50 and 55 0C, and in the model oils, the WAT was in the range of 50-60 0C. Only once there was lower WAT than 50 0C after the addition of the inhibitor, 26 wt% waxy oil with 1% of S-CD. All three inhibitors have one thing in common, and that is an increase in WAT in the model oils. There is no correlation whatsoever between the inhibitor concentration and different type of oils.

CONCLUSION
The proton NMR for M-CD showed very well defines the region of methyl group being attached to the one type of molecule, whereas the proton NMR for MS-CD suggested two distinct regions for -CH3 group. Moreover, the peaks in the proton NMR are not well defined as they are overlapping what could be expected as the mixture was composed of two different carboxylic acids with different chain length. The exact structure of the products hasn’t been described in detail. However, the structures could be analyzed by 2D NMR experiments such as COSY, HMQC, HMBC and mass spectrometry. All three synthesized compounds had different physical properties and the morphology of the crystals. The inhibitors had no significant effect on WAT in real oil samples, whereas in the model oil the WAT rapidly increased by the addition of the newly synthesized compounds. Data obtained by DSC were in a good correlation with the observations made by CPM. The results could be improved by additional steps required to lower WAT such as, use of the carrier solvent or adding less inhibitor into the oils. This work didn’t confirm the results which were achieved by Xue Yan in 2016. Although the data obtained in this work are not in agreement with the data from the papers, it is essentially the representation of the steps which are required if the papers are incomplete.
REFERENCES
- Sayers, R. (1983). WAX an Introduction. 1st ed. London: Genry Books Limited, pp.17-30.
- Wei, B. (2014). Recent advances on mitigating wax problem using polymeric wax crystal modifier. Journal of Petroleum Exploration and Production Technology, 5(4), pp.391-401.
- Wei, B., Li, H. and Xue, Y. (2016). A facile approach to synthesize a “star-like” material based on b-cyclodextrin for enhancing the flowability of paraffinic oils. Journal of Macromolecular Science, Part A, 53(7), pp.452-456.
- Wei, B., Li, H. and Xue, Y. (2016). Novel wax-crystal modifier based on b- cyclodextrin: Synthesis, characterization, and behavior in a highly waxy oil. Journal of Industrial and Engineering Chemistry, 43, pp.86-92.
- Chen, S., Fye, G. and Sjoblom, J. (2006). Characterization and Rheological Properties of Waxy oils. Annual Transactions of the Nordic Rheology Society, Vol. 14.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



