Stroke Diagnosis and Characterization
Abstract
Background: Stroke is one of the most burdensome neurological conditions affecting young and older adult populations, worldwide, and notably, it has remained one of the leading causes of disability and premature mortality within its disease category. Timely and accurate diagnosis of stroke with cross-sectional imaging impacts significantly on treatment planning and patient outcomes particularly with the advent of early reperfusion therapies. Recently, there has been growing recognition that perfusion-weighted MRI (PW-MRI) may offer a significant advantage in diagnosing and characterising the ischaemia induced by stroke as it can detect diffusion-perfusion deficits that may influence treatment decisions to prevent infarction. For researchers engaged in this field, healthcare dissertation help can provide valuable insights and support in exploring these advancements.
Aim: This structured literature review sought to explore the diagnostic utility and accuracy of perfusion-weighted MRI compared to other MRI protocols.
Methodology : A literature search was performed using MEDLINE, EMBASE and the Cochrane library and articles were limited to English language and publication in the last 15 years. Eligible articles were appraised using the QUADAS-2 framework and given marked inter-study heterogeneity, the diagnostic outcomes were analysed using a narrative approach.
Results: A total of 10 studies were reviewed; these were retrospective(n=9) and prospective(n=1) and found to observe a moderate to high risk of bias. The studies revealed that PW-MRI offers beneficial diagnostic capabilities when compared to other MRI sequences and the ability to detect significant diffusion-perfusion mismatches.
Discusion : However, PW-MRI was found to overestimate the degree of oligaemia and ischaemia in brain tissue. As ischaemia does not always progress to infarction, thrombolytic treatment may be unnecessary in some cases and expose patients to adverse effects.
Conclusion: These findings indicate that PW-MRI should be considered for routine imaging as an adjunct to other MRI protocols, also as an alternative to CT thereby reducing ionising radiation exposure.
Keywords: Perfusion-diffusion deficits; Cross-sectional diagnostic imaging.
INTRODUCTION
Stroke is a common and devastating neurological condition that primarily affects older people. It is one of the leading causes of permanent morbidity and mortality, with life expectancy averaging around the eighth decade of life1. Stroke is estimated to affect more than 13 million people worldwide each year22. Stroke has a lifetime risk of 25% and is the second leading cause of premature death after ischaemic heart disease4. Early reperfusion therapies, which rely on timely and accurate diagnosis as determined by cross-sectional imaging, have been the focus of efforts to reduce the burden of stroke.

The diagnosis of acute ischaemic stroke (AIS) is contingent on patient history, clinical examination findings and radiological evidence of cerebral vascular obstruction. Diagnosis in the first few hours of symptom onset has been informed by CT given its ready availability and high accuracy in detecting ischaemic and haemorrhagic change4. However, CT is associated with ionising radiation, which has known stochastic and deterministic effects upon long term health. It also has poor sensitivity in detecting early loss of grey-white matter differentiation, which can lead to false-negative diagnoses. CT, unlike MRI, is unable to provide information about the functional status of ischaemic brain tissue. MRI can provide detailed images of cerebrovascular perfusion, which is invaluable for understanding AIS pathophysiology and thus through more informed treatment, improved patient outcomes5.
However, the overall accuracy of MRI can vary depending on the sequences used. This could have an impact on decisions to prescribe brain tissue and life-saving reperfusion therapy6. Perfusion-weighted magnetic resonance imaging (PW-MRI) has been identified as one of the most promising MRI sequences22. This modality can assess the hemodynamic status of brain tissue and detect variations in perfusion that can aid in the identification, characterization, and differentiation of both the ischaemic core and the penumbra7.
The use of PW-MRI has recently increased in response to advances in reperfusion therapy and in particular, the approval of thrombolytic agents that have been one of the only treatments to improve AIS outcomes8. PW-MRI permits the ability to visualise blood flow and thus, perfusion of brain tissue, at the microvascular level. It is ideal for ascertaining the presence and extent of oligaemic, ischaemic and infarcted brain tissue. In DSM PW-MRI, the contrast administered leads to alterations in the magnetic field over time with the dephasing of spins, resulting in signal loss during perfusion. By applying a multi-phase acquisition, the perfusion of the contrast agent can be visualised over a temporal capture phase of 45-90 seconds and viewed as perfusion maps5. Notably, these maps also permit the quantification of perfusion parameters, such as the relative mean transit time and relative cerebral blood flow and volume. Numeric measures of perfusion may facilitate the characterisation of ischaemic change that can better inform salvageability and treatment in the future9. Evidence has shown that even minimal delays between CT and PW-MRI can demonstrate substantial variances in the extent of ischaemia and brain perfusion, which could cause clinical uncertainty10.
Take a deeper dive into Perfusion-Weighted MRI in Early Detection and Management of Ischemic Stroke with our additional resources.
The use of PW-MRI as complementary imaging to conventional MRI protocols has important clinical benefits. Parsons et al.11showed that the information concerning diffusion-perfusion mismatch attained using PW-MRI resulted in greater instigation and success of tPA therapy, compared to a historical control cohort who received DW-MRI. Treatment with tPA resulted in considerable sparing of infarcted brain tissue with a higher proportion of persons failing to progress to infarction. Furthermore, treatment resulted in considerably less expansion of infarct size, which would have accounted for significant variances in patient outcomes. Ryu et al.6conducted a meta-analysis of 13 trials exploring the utility of PW-MRI for stroke patients and found that it was more effective at informing patient suitability for reperfusion treatment as compared to other imaging. Patients receiving treatment following PW-MRI were 2-fold more likely to achieve a desirable neurological outcome at three months.
Clinical guidelines recommend that a non-enhanced CT is used to diagnose AIS should patients be suitable for thrombolysis. The acquisition time for CT is significantly faster than MRI and therefore more conducive to the acute environment. However, perfusion imaging using CT, or an MRI equivalent is essential for those who may be candidates for thrombectomy and presenting beyond six hours of symptom onset12,13. Presently MRI is reserved as a second-line imaging modality to that of CT for the diagnosis of AIS, although MRI offers the benefit of avoiding ionising radiation exposure and specific protocols it may provide superior imaging quality to permit accurate diagnosis4.
Comparison between DWS-MRI and PW-MRI
The interaction of free water protons with macromolecular protons in the prostate can be studied using conventional magnetization transfer (MT). Due to a substantial frequency offset, MT only provides a faint contrast between prostate cancer and benign areas65. By providing a smaller frequency offset, direct water proton saturation (DWS) may produce a stronger contrast as compared to the PW-MRI. Acute disease (ischemic stroke, cellular tumor, pus) frequently manifests as an increase in signal, indicating limited diffusion66. However, because the image contains a component generated from T2 signal, some tissues that are bright on T2 will appear bright on DWI imaging even if there is no abnormal limited diffusion.
Pros and cones of CT and MRI
Magnetic resonance imaging produces sharper images as compared to a CT scan. An MRI is preferable to x-rays or CT scans when clinicians need a view of soft tissues. When compared to CT56, MRIs can offer more accurate images of organs and soft tissues, such as torn ligaments and herniated discs. MRI scans cost roughly twice as much as CT scans. If money is a concern, talk to your doctor about if a less expensive imaging procedure might be performed. An MRI takes longer and may be too noisy for you, depending on your level of noise tolerance57. Some people experience anxiety or claustrophobia during an MRI because of the closed tube design and noisy operation. Any movement can result in blurred images56 because MRIs rely on precision to provide clear results. You must carefully control your breathing as recommended during the process. You may be able to breathe more freely if you need images of an area of your body that is not in your thoracic region, but you must remain perfectly motionless during the scan. MRIs also have the drawback of not being able to diagnose cancer 58. Cancer tissue and excessive fluids can appear the same on an MRI.
When compared to MRI scans, CT scans have several advantages. CT scans may be a better option for larger individuals who may not fit comfortably inside traditional MRI machines due to their more open design 57. Because this procedure produces results much faster than an MRI, doctors prefer it as a scanner for making an emergency diagnosis. When it comes to determining the cause of a stroke and initiating treatment, a CT scan cannot compete59. Doctors can determine whether the stroke was caused by haemorrhaging or by a blocked artery. While CT has advantages, it is not without flaws. A computed tomography scan can expose you to up to 1,000 times the amount of radiation that an x-ray exposes you to 56. Even at these levels, the radiation is a low dose, but if you need multiple scans over the course of your life, it can add up. Despite being less expensive, CT images do not capture as many details as MRIs, and the doctor may miss important information 60. While larger patients can be accommodated by CT machines, there is still a limit. Depending on the model, traditional scanners can accommodate patients weighing up to 450 pounds. The patient's back-to-front measurement across the widest point should be less than 28 inches as well59.
What your doctor is looking for will determine which scan is best for you. Your symptoms and medical history will aid your doctor in making the best diagnostic scan choice. When your doctor determines the optimum scan for you, the risk factors that may prevent you from having a CT scan or an MRI will be taken into account. Although MRI scans are low-risk, they are not appropriate for everyone 61. The MRI's powerful magnetic field may cause issues for those who have metallic implants in their bodies. If you have an IUD, artificial joints, a pacemaker, or ocular implants, talks to your health care provider about whether an MRI is essential.
Search Strategy
The central research question for this SLR was derived utilizing the suggested PICO framework14, 15. Its goal was to assess the diagnostic accuracy, sensitivity, specificity, and predictive values of PW-MRI and other MRI protocols in adults for the diagnosis of AIS, determine the implications for clinical practice and guidelines, and propose topics for future research. Methods A search query was created and applied in the PubMed and SCOPUS databases. The following search phrases were obtained from the review question and the informative PICO elements: Intervention: perfusion-weighted MRI, comparator: other MRI protocols, and outcomes: diagnostic accuracy. A scoping study helped identify new phrases that would increase searchability16, ensuring that all appropriate terms were used. Using Boolean operators like ‘OR' and ‘AND,' the final terms were combined into a string. The comparator component was excluded from the search method (table 1) because its inclusion could have resulted in excessive search precision and a significant chance of missing relevant studies18.Studies with a relevant comparator of congruence to the review question were identified during study filtering and selection.
Inclusion and Exclusion Criteria
Table 2 shows the inclusion criteria, which were based on the PICO elements. The previously mentioned diagnostic accuracy indices were chosen as the major outcomes since they are extensively used in the literature to characterize the accuracy of diagnostic tests, albeit due to the small number of relevant studies, diagnostic utility was also taken into account19. Furthermore, image interpreter reports on the utility and correctness of MRI procedures were taken into account, as this might provide a realistic and pragmatic viewpoint on radiological tests20. Notably, the comparator component of diagnostic accuracy reviews involving imaging tests would usually seek to compare the accuracy with the gold reference standard, which is normally histological examination21. However, histology was not apposite. Thus, the comparator was deemed to be non-PW-MRI protocols.
The results of the study search and selection are described and presented using the standard PRISMA format (Fig 1.).
Data Extraction and Analysis
Study data was retrieved onto pre-defined templates using a rigorous and reliable process12, and these were transcribed into physical and electronic databases to allow for inter-study comparisons. The diagnostic accuracy data from each trial were summarized using a tabular technique, and the overall diagnostic accuracy of PW-MRI was computed using the mean sensitivity, specificity, NPV, and PPV, among other indices. Sensitivity and specificity were estimated from the raw data of informing studies using the equations provided in Table 3 or as a mean of those presented. Similarly, the mean negative predictive value and positive predictive value were calculated using the following equations: NPV = true negatives / (false negatives + true negatives) and PPV = true positives/ (true positives + false positives)24.
RESULTS
Following search and selection, 10 studies the inclusion/exclusion criteria. The search results can be seen in Figure 1.
The collective sample size was 1,184 subjects however there was wide variance across the included studies. The summary of study characteristics can be seen in Table 4. The majority of these studies26,27,29,30,31,32,35 did not define the power attained for the sample sizes analysed and thus, those with low sample sizes could have been at risk of type II error 36, although the lack of reporting makes this inference unclear. In contrast, three studies observed sample sizes exceeding 100 subjects and thus, a low risk of type II error was inferred28,33,34
All included studies explored the diagnostic utility and/or accuracy of PW-MRI, although there were interstudy-variances in terms of the specific technical protocols used to acquire images and comparator imaging26-35. All studies compared PW-MRI against the appropriate comparator of diffusion-weighted MRI, three of these also compared the protocol with additional MR sequences including MR angiography28,29 and fluid-attenuated inversion recovery: FLAIR33. Three studies failed to define the specific technical parameters used to acquire perfusion- and diffusion-weighted MR images and thus, it is not possible to compare the diagnostic utility/accuracy of these protocols with other studies in this review30,32,33. All studies utilised contrast-enhanced MRI with a magnetic field strength of 1.5 Tesla, although one study included a mix of MR scans taken at 1.5 and 3.0 Tesla, which may have implications for the diagnostic utility33.
Among studies included in this review, there was varied reporting of the diagnostic utility and accuracy of PW-MRI and other MR protocols. The specific outcomes reported included sensitivity, specificity and/or inter-observer variability27,30,33,34, the degree of perfusion-diffusion mismatching28,29,30,31,32,35, lesion size or extent26,32 and treatment suitability and clinical outcomes28,29,31,32,33. QUADAS-2 quality assessment
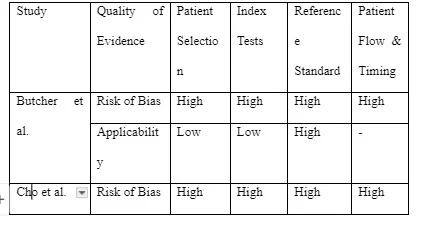
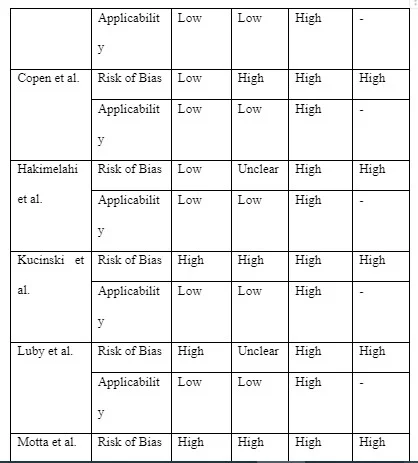
The outcomes from the quality assessment are detailed in Table 5. Overall, the risk of bias was deemed to be high and concern over applicability was also high. This is not unexpected given all studies employed retrospective methodologies. A major contributor to these findings was the use of DW-MRI as a reference test. No histological gold standard is readily available and thus the risk of reference misclassification bias38 was deemed high given that diffusion-weighted imaging is not completely sensitive and specific for ischaemic stroke.
Diagnostic Utility and Accuracy of PW-MRI The reporting of the diagnostic utility and accuracy of PW-MRI varied across studies included in this review due to the various measures that were employed to ascertain the diagnosis of ischaemic stroke and/or to characterize the extent of ischaemia. Firstly, four studies reported upon the usually accepted measures of diagnostic accuracy, which include sensitivity and specificity27,30,33,34. Butcher et al.27 showed that PW-MRI observed a sensitivity of 78% and a specificity of 88% for eliciting perfusion-diffusion mismatches by volume and with highly inter-rated reliability with kappa scores exceeding 0.94 for experienced radiologists. However, the agreement was much less in inexperienced radiologists (kappa 0.28-0.49). Despite this, PW-MRI was significantly better at detecting perfusion-diffusion mismatching, as compared to DW-MRI Simonsen et al.33 showed that the sensitivity and specificity of DW-MRI for diagnostic ischaemic stroke were 92% and 75%, respectively, however, complementing the protocol with PW-MRI increased the sensitivity further to 97.5%. The authors also explored the effect of combined MRI scanning upon clinical outcomes, albeit indirectly, where one patient had a negative DW-MRI scan but a positive perfusion-weighted scan and this resulted in a large infarction and a poor neurological outcome. Notably, the poor specificity of DW-MRI was largely observed for persons with PCS, which was significantly likely to be the location of ischaemia when imaging was negative (p=0.0019). Straka et al.32 also explored the utility of combined diffusion-perfusion MRI using an automated RAPID protocol to calculate diffusion-perfusion mismatching. The results showed that DW-MRI observed a slightly greater correlation than PW-MRI for acute stroke (0.99 v. 0.96), although the difference was not significant. Overall, the sensitivity and specificity of combined imaging were reported as 100% and 91%, respectively, which is similar to the accuracy reported formerly. Wolman et al.34 even showed that DW-MRI and PW-MRI provided a comparable sensitivity (95.9%) and specificity (98.4%) for the accurate triage of patients with ischaemic stroke to endovascular therapy and notably, this imaging approach was associated with successful recanalization of the obstructed cerebral vasculature in 96% of cases. Several of the other studies also reported upon the ability of PW-MRI to characterise perfusion-diffusion mismatches. In one study, Cho et al.28 showed that among 52 patients with ACS, three cases observed negative DW-MRI scans but marked perfusion abnormalities on PW-MRI, which represents a 100% mismatch and indicates the marked clinical utility of perfusion imaging. Similarly, Hakimelahi et al.35 also revealed that a complete 100% diffusion-perfusion mismatch was observed between the respective MRI protocols in 49 of 68 patients with lesion volumes in the retrospective component and 35 of 48 patients in the prospective cohort . An earlier study8 revealed that the images from PW-MRI provided vital detail of regions of low perfusion and oligaemia and thus, areas that were at risk of infarction which could be potentially spared using thrombolytic therapy. Such areas of ischaemic compromise were not evident when using DW-MRI, suggesting that this protocol would lead to under-diagnosis and under-treatment of some cases particularly as the diffusion-perfusion mismatch persisted for more than nine hours in 84% of cases. In one case, PW-MRI was able to elicit a 340% mismatch at almost 6-hours post-onset of stroke symptoms, as compared to DW-MRI that would have missed the extent of ischaemic tissue. Overall, PW-MRI was able to identify 20-50% mismatches even when DW-MRI observed areas of cerebral hypoperfusion.
Whilst PW-MRI has been consistently supported as a highly useful imaging modality for ischaemic stroke, the findings of Kucinski et al.26 demonstrate the contrary where the protocol was associated with over-estimating the extent of hypoperfused brain tissue at risk of infarction. This showed that the mean volume of cerebral blood that did not progress to infarction was 148ml but the sensitivity for predicting infarction death was only 56%. Similarly, Motta et al.32 showed that the degree of perfusion observed on PW-MRI did not always correlate with cognitive deficits in patients and persistent hypoperfused areas of the brain did not always progress to ischaemia and infarction, suggesting that the protocol is prone to over-diagnosis or over-characterization of ischaemic stroke. However, the utility of PW-MRI for providing a measure of reliability for mismatching pre- and post-thrombolytic therapy has been demonstrated to be desirable by Luby et al.31 where the sensitivity, specificity and PPV were 82%, 80% and 81%, respectively.
Discussion
The QUADAS-2 assessment revealed that 60% of the 10 studies observed a high risk of bias for the patient selection and index test domains and 100% observed a high risk of bias for the reference standard domain and patient flow and timing.
Through this process of narrative description, the results revealed that in most cases, PW-MRI offers superior diagnostic capabilities when compared to DW-MRI and MR angiography with a sensitivity as high as 100% and the ability to detect significant diffusion-perfusion mismatches, which can significantly influence changes to management and in turn, neurological outcomes. However, it was identified that PW-MRI may over-estimate the degree of oligaemia and ischaemia in brain tissue and indeed, temporal observations showed that such aberrations did not always progress to overt infarction. Thus, treatment in such cases would be unwarranted and expose patients to unnecessary effects. Despite this, it was demonstrated that PW-MRI could substantially benefit the care for a select number of cases including the better characterisation of posterior circulation strokes, a limiting factor of DW-MRI. It also demonstrated consistency in detecting 20-50% mismatches and in a proportion of cases, mismatches in the order of 340%.
In view of the diagnostic accuracy of PW-MRI where a high sensitivity for AIS (97.5%) was observed, this suggests that the likelihood of the correct diagnosis or classification of stroke even when changes in brain tissue are markedly subtle is high and the risk of a false negative is low. However, the literature demonstrated that sensitivity varied somewhat, being as low as 78% for detecting perfusion-diffusion mismatches29. However, the low sample size of this study may have resulted in an underestimation of the true accuracy of PW-MRI. Overall PW-MRI observed a specificity value of 75-88%. This appears to be largely due to difficulties in discriminating AIS from stroke mimics e.g. migraine and alcohol intoxication33. Unfortunately, this detection and interpretation of perfusion-diffusion mismatches led to the treatment of stroke mimics with unnecessary thrombolytic therapy only recognised following a more stringent review of the clinical and image data. This problem may be difficult to overcome in practice particularly given the narrow window of opportunity to instigate brain-sparing stroke therapy and clinical decision making that likely favours the treatment of AIS even when a possibility of a stroke mimic exists, as a lack of treatment could be disabling or fatal in actual AIS cases.
Evidence to support the diagnostic accuracy indices of PW-MRI for AIS was limited by the lack of wider research reporting upon these outcome measures. Despite this, a recent meta-analysis of the accuracy of conventional MRI showed that the protocol has a similar sensitivity and specificity to that reported for PW-MRI here but indicated values that were superior to CT39. These observations have also been supported in another meta-analysis of the accuracy of CT and MRI for AIS40. Previous evidence has supported the superior ability of PW-MRI in characterising diffusion-perfusion mismatches when compared to DW-MRI findings7,41,42. Notably, such diffusion-perfusions deficits can persist for more than nine hours, exceeding the guideline-recommended treatment threshold by two-fold. Thus, for patients presenting with late-onset AIS, PW-MRI may offer diagnostic information that could signal suitability for reperfusion therapy8. Copen et al.8 believe that the ambiguity of AIS symptoms and thus, time of presentation to clinicians is due to inter-patient differences in the extent of collateral cerebral circulation. In those with extensive collateral supply and thus, a large diffusion-perfusion deficit, patients are likely to experience milder neurological deficits, whilst those with the poorer collateral flow and thus, small diffusion-perfusion deficits are likely to observe more significant neurological impairment. Thus, the former is likely to present late, and the latter is likely to present sooner after AIS onset. However, in either case, the ability of PW-MRI to more accurately characterise mismatches in diffusion and perfusion appears to represent the ideal imaging modality for informing AIS treatment.
In the hyperacute phases of AIS, imaging using CT is the first-line modality for imaging the brain given the ready availability of scanners and fast acquisition times, which are desirable to inform reperfusion therapy given that delays in treatment progressively increase the risk of poorer outcomes43. However, some studies have shown that whilst conducting MRI can take additional time, the neurological outcomes of patients who subsequently receive reperfusion therapy are similar44-47. The use of MRI is associated with a significantly reduced rate of symptomatic stroke post-therapy45 suggesting that the risks of delayed treatment do not outweigh the benefit of attaining additional imaging information to directly inform therapy. The National Clinical Guidelines for Stroke48 recommend that reperfusion therapy is commenced as soon as possible after the ascertainment of AIS but within 3-4.5 hours of symptom onset and thus, opting to utilise MRI instead of CT to inform therapy is a reasonable and achievable option48.
The findings of this review suggest that the use of perfusion-weighted imaging should supersede that of diffusion-weighted imaging as this is likely to better inform therapeutic decision making and in turn, patient outcomes. The primary reason for the benefit of PW-MRI is due to its superior ability to characterise diffusion-perfusion mismatches. According to the mismatch hypothesis, the difference in diffusion and perfusion of blood to an area of brain tissue provides vital information concerning the reversibility of ischaemia and thus, the time window of opportunity to intervene and optimise the likelihood of preventing irreversible infarction damage49. Indeed, the landmark DEFUSE and EPITHET trials50,51 showed that the profiling of diffusion-perfusion mismatching in patients with AIS and the instigation of early reperfusion therapy in these individuals resulted in significantly more favourable outcomes, as compared to patients who did not receive PW-MRI to provide information about mismatching (p<0.01).
The findings of this review and other studies evaluating the modality in isolation 52 indicate that DW-MRI was associated with a considerable false-negative rate. Specifically, this review showed that PW-MRI can increase the sensitivity of diffusion-weighted imaging from 90% to 100% and thus, this reflects a 10% lower risk of false-negative diagnosis. At present, however, PW-MRI is not considered a routine MRI protocol and thus, DW-MRI remains the first-line approach and where negative, PW-MRI is employed to improve certainty in the exclusion or confirmation of stroke48,53. Most studies in this review utilised magnetic field strengths of 1.5 Tesla with only Simonsen et al.35using a mix of 1.5 and 3.0 Tesla. Rosso et al.54have previously confirmed that 1.5 Tesla offers greater sensitivity for stroke, as compared to 3.0 Tesla (99.1% v. 92.5%) as well as conferring a lower false-negative rate (0.6% v. 6.1%). As the magnetic field strength was the only considerable technical variant among the MRI protocols of studies included in this review, any recommendations for PW-MRI should consider the use of 1.5 Tesla, although this requires evaluation in future research. However, the diagnostic utility of PW-MRI can also be affected by other limitations including subjectivity and related bias regarding the interpretation of perfusion maps and the various techniques that can be used to generate quantitative measures of perfusion status, which has been known to confer significant variances in ischaemia volume in some cases9. Perfusion-weighted imaging can result in over-estimations of diffusion-perfusion mismatches, albeit to a lesser extent than diffusion-weighted imaging, although in other cases, the extent of tissue at risk of infarction may be under-estimated9. Efforts to try and resolve this problem have focused upon defining thresholds of mismatching but this has been compromised due to inter-patient variances and the course of AIS, which means that it is unlikely that universal thresholds for mismatching to inform treatment will emerge in the future27. Thus, the images provided by PW-MRI in patients with AIS will probably have to be considered on a case-by-case basis if treatment decision making is to be safely and optimally informed.
Overcoming the specific limitations of different MRI protocols may however be achieved through utilising multimodal MRI strategies that comprise the simultaneous acquisition of diffusion-weighted, perfusion-weighted, gradient-echo, angiography and FLAIR MRI, as has been suggested by Kim et al.22. Although there may be some concern that the acquisition times of multimodal MRI may narrow the window of opportunity to instigate reperfusion therapy suggested in clinical guidelines, there is now sufficient evidence that MRI protocols can be acquired within a reasonable period that is not excessively disparate to CT. For some patients, MRI or gadolinium contrast agents are contraindicated i.e. those with metal implants or with severe renal problems but for those who are suitable, this could extend the treatment eligibility for patients with AIS and in turn, lead to improvements in neurological outcomes22,55. Advantages and disadvantages of using MRI and CT scan A CT scan, also known as a CAT scan, is a non-invasive diagnostic tool that allows your doctor to inspect your organs, soft tissue, and bones. The most significant advantage of a CT scan is that it is often significantly faster than an MRI and costs around half as much 62. This imaging approach is particularly good at catching bone structure details, but it can also capture soft tissue and blood arteries. Furthermore, unlike an MRI, many people find that CT scans have the advantage of rarely causing claustrophobia during the process 63. However, like with every diagnostic examination, there are potential disadvantages. The most significant disadvantage of a CT scan is that it emits radiation. Although the amount of radiation is relatively low, repeated exposure is not suggested, and pregnant women should avoid CT scanning 61. The fact that MRI machines do not release radiation makes them safe for pregnant women is a significant advantage of this treatment 64. This diagnostic technique can create detailed images in any plane, but it works best with soft tissue detail. MRIs, like CT scans, have several disadvantages. They are more adaptable than x-ray equipment, for example, although they yield less detailed results 60. Additionally, because the treatment is performed in a small, enclosed location, some people may experience anxiety throughout it.
 Conclusion
Conclusion
This study revealed that PW-MRI offered beneficial diagnostic capability for diagnosing AIS, compared to DW-MRI and MR angiography for AIS. Given that PW-MRI tends to provide superior accuracy for diagnosing and characterising AIS, it is recommended that this protocol is used in place of or as an adjunct to DW-MRI. PW-MRI permits the calculation of diffusion-perfusion mismatches, which is essential for detecting penumbrae and in turn, predicting regions of brain ischaemia at risk of infarction. This is fundamental to informing both acute and delayed reperfusion therapy. Current guidelines should seek to incorporate recommendations regarding the utility of PW-MRI for patients presenting with signs or symptoms of AIS within and beyond the 4.5-hour threshold for thrombolytic or thrombectomy treatment. MRI in hyperacute and AIS has been shown to have comparable diagnostic accuracy and unlike CT or standard MRI protocols alone, can provide clinicians with additional data around the functional status of ischaemic brain tissue to better inform their treatment plans and thus positively impact patient outcomes. In patients presenting early with AIS, standard MRI with PW-MRI should be considered as a first-line imaging modality, as greater characterisation of brain oligaemia and ischaemia may lead to more appropriate decision-making regarding reperfusion therapy.
Looking for further insights on Stress Continence in Women? Click here.
REFERENCES
Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol [online]. 2019;18(5):417-418.
Campbell BCV, De Silva DA, MacLeod MR, et al. IschaemicStroke. Nature Reviews Disease Primers. 2019;5(1):70-78.
WHO. 2018. The top 10 causes of death [online].
Bal S, Bhatia R, Menon BK, et al. Time Dependence of Reliability of Noncontrast Computed Tomography in Comparison to Computed Tomography Angiography Source Imagein Acute Ischemic Stroke. Int J Stroke [online]. 2015;10(1):55-60.
Harris AD, Coutts SB, Frayne R. Diffusion and perfusion MR imaging of acute ischemic stroke. Magn Reson Imaging Clin N Am. 2009;17(2):291-313.
Ryu WHA, Avery, MB, Dharampal N, et al. Utility of perfusion imaging in acute stroke treatment: a systematic review and meta-analysis. J Neurointerv Surg [online]. 2017;9(10):1012-1016.
Neumann-Haefelin T, Wittsack, HJ, Wenserski F, et al. Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke [online]. 1999;30(
8):1591-1597.
Copen WA, Schaefer, PW, Wu O. MR perfusion imaging in acute ischemic stroke. Neuroimaging Clinics of North America. 2011;21,(2):259-267.
Kane I, Carpenter, T, Chappell F, et al. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke: effect on lesion size, proportion of patients with diffusion/perfusion mismatch, clinical scores, and radiologic outcomes. Stroke [online]. 2007;38(12):3158-3164.
Campbell BC, Christensen S, Levi CR. Et al. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic
stroke. Stroke. 2012;43(10):2648-2653.
Parsons MW, Brber, PA, Chalk J. et al. Diffusion‐ and perfusion‐weighted MRI response to thrombolysis in stroke. Ann Neurol [online]. 2002;51(1);28-37.NICE. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. 2019. [online].
White P, Nanpragasam A. What is new in stroke imaging and intervention?.Clinical Medicine (London, England) [online].2018;18:13-16.
Pollock A. Berge E. How to do a systematic review. Int J Stroke [online]. 2018;13(2):138-156.Farrugia P, Petrisor BA, Farrokhyar F. et al. Research questions, hypotheses and objectives. Canadian Journal of Surgery. 2010;53(4):278.
Maltby J, Williams G, McGarry J et al. Research methods for nursing and healthcare. 2014. Routledge.
Bettany-Saltikov J. How to do a systematic literature review in nursing: a step-by-step guide. 2012. McGraw-Hill Education (UK).Aromataris E. Riitano D. Constructing a search strategy and searching for evidence. A guide to the literature search for a systematic review. Am J Nurs [online].
2014;114(5):49-56.
Simundic, AM, Measures of Diagnostic Accuracy: Basic Definitions. Ejifcc, 2009;19,(4):203-211. Leeflang MM, Deeks JJ, Gatsonis C et al.Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149(12):889-897.Schmidt RL, Factor RE, Understanding Sources of Bias in Diagnostic Accuracy Studies. Arch Pathol Lab Med [online]. 2013;137(4):558-565.
Researchers-Part I. General Guidance and Tips. Korean J Radiol. 2015;16,(6):1175-1187.
Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quali assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8);529-536.online]. 2008;56(1):45-50.
Moher D, Liberati A, Tetzlaff, J et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoSMedicine. 2009;6(7).
Kucinski T, Naumann D, Knab R, et al. Tissue at Risk Is Overestimated in Perfusion-Weighted Imaging: MR Imaging in Acute Stroke Patients without VesselRecanalization. American Journal of Neuroradiolog. 2005;26,(4):815-820.
Butcher K, Passons M, Allport L, et al. Rapid assessment of perfusion-diffusion mismatch. Stroke [online]. 2008;39(1);75-81.
Cho T, Hermier M, Alawneh, JA, et al. Total mismatch: negative diffusion-weighted imaging but extensive perfusion defect in acute stroke. Stroke [online].
2009;40(10):3400-3402.Copen, WA, Rezai Gharai L, Barak ER, et al. Existence of the diffusion-perfusion mismatch within 24 hours after onset of acute stroke: dependence on proximal
arterial occlusion. Radiology [online]. 2009;250(3);878-886.
Straka M, Albers, GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging [online]. 2010;32(5):1024-1037.
Luby, M, Ku KD, Latour LL, et al. Visual perfusion-diffusion mismatch is equivalent to quantitative mismatch. Stroke [online]. 2011;42(4),1010-1014.Motta M, Ramadan A, Hillis , et al. Diffusion-perfusion mismatch: an opportunity for improvement in cortical function. Frontiers in Neurology. 2015;5(1):280-290.
Simonsen CZ, Madsen MH, Schmitz ML, et al. Sens/itivity of Diffusion- and Perfusion-Weighted Imaging for Diagnosing Acute Ischemic Stroke Is 97.5. Stroke [online].2015;46(1):98-101.
Wolman DN, Iv M, Wintermark M, et al. Can diffusion- and perfusion-weighted imaging alone accurately triage anterior circulation acute ischemic stroke patients to endovascular therapy?.J Neurointerv Surg [online]. 2018;10(12):1132-1136.
Hakimelahi R, Yoo, AJ, He J, et al. Rapid identification of a major diffusion/perfusion mismatch in distal internal carotid artery or middle cerebral artery ischemic stroke. BMC Neurol [online]. 2012;12(1):132.
Nayak B, Understanding the relevance of sample size calculation. Indian J Ophthalmol [online]. 2010;58(6):469-470.
Martinez-Mesa J, Gonzalez-Chica DA, Duquia RP, et al. Sampling: how to select participants in my research study?.Anais Brasileiros De Dermatología; an BrasDermatol [online]. 2016;91(3):326-330.
Biesheuvel, C, Irwig L, Bossuyt P, Observed Differences in Diagnostic Test Accuracy between Patient Subgroups: Is It Real or Due to Reference Standard Misclassification?.Clin Chem [online]. 2007;53(10):1725-1729.
Zhang X, Liang, H. Systematic review with network meta-analysis: Diagnostic values of ultrasonography, computed tomography, and magnetic resonance imaging in
patients with ischemic stroke. Medicine (Baltimore) [online]. 2019;98(30):16360.
Xin Y, Han FG.Diagnostic accuracy of computed tomography perfusion in patients with acute stroke: A meta-analysis. J Neurol Sci. 2016;360(1):125-130.
Sorensen AG, Copen WA, Ostergaard L, et al. Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and meantissue transit time. Radiology [online]. 1999;210(2)519-527.
Darby DG, Barber PA, Gerraty RP, et al. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke [online].
1999;30(10):2043-2052.Kang D, Chalela JA, Dunn W, et al. MRI screening before standard tissue plasminogen activator therapy is feasible and safe. Stroke [online]. 2005;36(9):1939-1943.
analysis of 1210 patients. Stroke [online]. 2007;38(10):2640-2645.
Köhrmann M, Jüttler, E, Fiebach, et al. MRI versus CT-based thrombolysis treatment within and beyond the 3 h time window after stroke onset: a cohort study. Neurol [online]. 2006;5(8):661-667
Royal College of Physicians. National clinical guideline for stroke [online]. 2016.
Bateman M, Slater L, Leslie-Mazwi T, et al. Diffusion and Perfusion MR Imaging in Acute Stroke: Clinical Utility and Potential Limitations for TreatmentSelection. Top Magn Reson Imaging [online]. 2017;26(2):77-82.
Albers GW, Thijs VN, Wechsler L, et al. 2006. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusionimaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol [online]. 2006;60(5)508-517.
Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol [online]. 2008;7(4):299-309.
Oppenheim C, Stanescu R, Dormont D, et al. False-negative diffusion-weighted MR findings in acute ischemic stroke. Am J Neuroradiol. 2000;21(8):1434-1440.
Powers WJ, Rabinstein A, Ackerson, T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for HealthcareProfessionals From the American Heart Association/American Stroke Association. Stroke. 2018;49,(3):46-99.
Rosso C, Drier A, Lacroix D, et al. Diffusion-weighted MRI in acute stroke within the first 6 hours: 1.5 or 3.0 Tesla?.Neurology [online]. 2010;74(24):1946-1953.
Nael K, Khan R, Choudhary G, et al. Six-Minute Magnetic Resonance Imaging Protocol for Evaluation of Acute Ischemic Stroke: Pushing the Boundaries. Stroke [online]. 2014;45(7):1985-1991.
Kim BJ, Kang HG, Kim H, et al. Magnetic resonance imaging in acute ischemic stroke treatment. J Stroke [online], 2014;16(3):131-145. Burnier M, Fricker AF,
Hayoz D, et al. Pharmacokinetic and pharmacodynamic effects of YM087, a combined V1/V2 vasopressin receptor antagonist in normal subjects. Eur J ClinPharmacol.1999;55:633–637.
Ladd, Mark E., et al. "Pros and cons of ultra-high-field MRI/MRS for human application." Progress in nuclear magnetic resonance spectroscopy 109 (2018): 1-50.Servello, Domenico, et al. "The pros and cons of intraoperative CT scan in evaluation of deep brain stimulation lead implantation: A retrospective study.
" Surgical neurology international 7.Suppl 19 (2016): S551.Hascoët, Sebastien, et al. "Cardiac imaging of congenital heart diseases during interventional procedures continues to evolve: pros and cons of the main techniques." Archives of cardiovascular diseases 109.2 (2016): 128-142.
Kapur, Savinay, Ashu Seith Bhalla, and Manisha Jana. "Pediatric chest MRI: a review." The Indian Journal of Pediatrics (2019): 1-12.
Visentin, Sindi, et al. "Post-autopsy computed tomography. Pros and cons in a firearm death." Forensic science international 276 (2017): e16-e19. Yang, Y., He, M. Z., Li, T., & Yang, X. (2019). MRI combined with PET-CT of different tracers to improve the accuracy of glioma diagnosis: a systematic review andmeta-analysis. Neurosurgical review, 42(2), 185-195.
Xiong, Yunyun, et al. "Comparison of automated CT perfusion softwares in evaluation of acute ischemic stroke." Journal of Stroke and Cerebrovascular Diseases 28.12 (2019): 104392.
Sergi, Giuseppe, et al. "Measurement of lean body mass using bioelectrical impedance analysis: a consideration of the pros and cons." Aging clinical and
experimental research 29.4 (2017): 591-597.
Stephen, Joanna M., et al. "Comparative accuracy of lower limb bone geometry determined using MRI, CT, and direct bone 3D models." Journal of Orthopaedic
Research® 39.9 (2021): 1870-1876.
Bollache, Emilie, et al. "Comparison of 4D flow and 2D velocity-encoded phase contrast MRI sequences for the evaluation of aortic hemodynamics." The international
journal of cardiovascular imaging 32.10 (2016): 1529-1541.
Qin, Jiang-bo, et al. "Grading of gliomas by using radiomic features on multiple magnetic resonance imaging (MRI) sequences." Medical science monitor: international medical journal of experimental and clinical research 23 (2017): 2168.
Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol [online]. 2019;18(5):417-418.
Campbell BCV, De Silva DA, MacLeod MR, et al. IschaemicStroke. Nature Reviews Disease Primers. 2019;5(1):70-78.
APPENDIX
Table 1. Search Summary
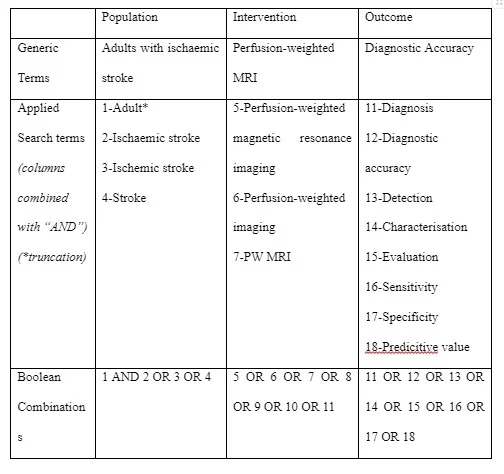
Table 2. Inclusion and exclusion criteria
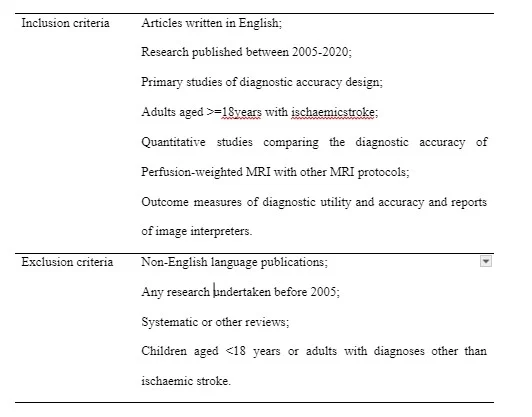
Table 3. Calculation of sensitivity and specificity26
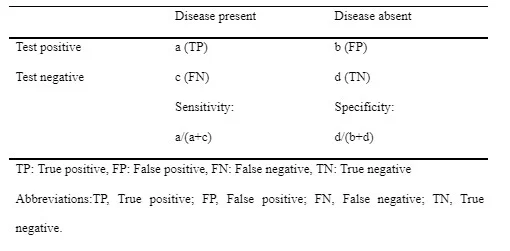
Table 4. Summary of study characteristics

Table 5. Summary of risk of bias using QUADAS-2.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



