To Explore Perceptions about weight gain
1. Background
A kidney transplant, also known as a renal transplant, is a surgical procedure that replaces a diseased kidney with a healthy kidney from a donor. When both kidneys cease working properly, the body is unable to filter the blood and maintain fluid balance, which necessitates a renal transplant.(Hollis et al., 2017). After renal transplant, patients express increased risk of development of type-2 diabetes and weight gain. This is because of side-effects of different medication, physical health condition, diet management, lifestyle actions and others (Issa et al., 2018). In renal transplant patients, the rise of diabetes may occur even if the patients did not express any pre-diabetic symptoms or does not have any family member who have previously suffer from diabetes (Rysz et al., 2021). The study by Gaiffe et al. (2019) mentions that new onset of diabetes after renal transplants affects nearly 15-20% of patients and it is related with increased morbidity and lower survival efficiency of the transplant patients as well as the transplants itself. In the study by Patel et al. (2016), it is mentioned that 7-30% of the patients with renal transplant express risk of new onset of diabetes after the transplant. This indicates that rise of type-2 diabetes in renal transplant patient after surgery is common, and seeking healthcare dissertation help may provide further insights into managing these risks effectively.

The study by Gaiffe et al. (2019) informs that the onset of diabetes after renal transplant act as risk factor for the development of atherosclerotic event in patients. The study also mentions that corticosteroids, mammalian target of rapamycin (mTOR) and anti-calcineurin act to create key diabetogenic effects and greatly contribute towards the increased prevalence of diabetes in transplant patients (Gaiffe et al., 2019). It indicates that rise of diabetes after the transplant leads to development of additional comorbid diseases which could act to hinder the health and well-being of the individuals. In the study by Liaveri et al. (2017), it is mentioned that before and after the renal transplant, the body undergoes the experience of increased stress and trauma which is responsible in raising the blood sugar levels. It is evident as before the transplant, the patients undergo immense stress regarding the health and performance of the body to accept the trauma to be faced with transplantation. Moreover, after the transplant, the patients are required to take increased dose of medication which creates additional stress on the body. The added stress in renal transplant patients makes the body prepare itself by producing increased blood sugar to allow enhanced amount of energy is present in the body to overcome the stress (Nie et al., 2019).
The study by Boerner et al. (2014) mentions that renal transplant patient are required to take increased amount of immunosuppressant’s in managing their health. This is because during transplant, the new donor kidney considered foreign by the body during transplant, the immune system will reject it and destroy the cells. However, with presence of immunosuppressants, the immune system of the of the body is supressed to detect the donor kidney as foreign and support its acceptance to allow working in the body. As argued by Yepes-Calderón et al. (2019), immunosuppressant’s in renal transplant patients mainly act to reduce the performance of the immune system which decreases the performance of the body to use insulin. In this condition, the body express insulin resistance that account for rise on blood sugar level and type-2 diabetes development in renal transplant patients. In contrast, the study by Nolte Fong and Moore. (2018) mentions that steroids provided to renal transplant patients trigger their body to respond in different way to food intake such as carbohydrates. The carbohydrates are mainly involved in supporting healing, repairing and providing energy to the body to cope with the condition. In transplant patients, they intend to take increased carbohydrates to support enhanced presence of energy which also triggers the body to raise the sugar level. The rise of sugar level may be uncontrolled in transplant patient due to their hindered diet which result the patients be at risk of develop diabetes.
The study by Gaiffe et al. (2019) mentioned that few pharmacological preventions are found for managing diabetes in renal transplant patients. For instance, immediate administration of short-term insulin to renal transplant patients after the surgery is found to reduce the incidence of diabetes progression in one-year of transplant. The occurrence of diabetes in this condition is regarded as secondary endpoint and it is found to be reduced by 73% in the group treated with insulin. Moreover, other relevant experiments have suggested use of gliptins is useful in the pharmacological prevention of diabetes in renal transplant patients. This is because they create protective impact on the β cells of the pancreas and support anti-inflammatory actions which cause delay or prevention of occurrence of diabetes after the renal transplant in patients (by Gaiffe et al., 2019). The study by Forte et al. (2020) informs that 50% of the patients after renal transplant express increased weight gain apart from diabetes after the surgery irrespective of the pre-transplant nutritional status of the individual. Similarly, the study by Orazio et al. (2014) mentioned that after the first year of renal transplant, the patients experience significant increase in weight and obesity with their average weight gain leading to 10-35% of the body mass and bone mineral density. The changes are mentioned to be related with increased fat in the body, reduced bone mineral density and lower lean muscle mass. The increased weight gain in the initial year is found to support development of diabetes and other metabolic disorder in renal transplant patients (Orazio et al., 2014). This indicates that weight gain is common risk after the renal transplant in patients.
The study by Kluch et al. (2020) mentioned that weight gain in renal transplant patients are caused by side-effects of immunosuppression medications which is similar cause that supports development of diabetes in the patient. Thus, the medication acts as independent risk for development of weight gain and diabetes in renal transplant patients. In the study by Sabbatini et al. (2019), it is mentioned that relaxation of the dietary restrictions related with dialysis and improvement in the appetite-related with resolution of uremic state contributes to the weight gain in renal transplant patients. This is because the liberal nature of food intake by the renal transplant patients makes them include increased food in the diet which adds to their body weight. The immosupressive medication also contributes to increase the appetite of the patients which makes them intake high amount of carbohydrates and fat in the form of food leading them to face weight gain and type-2 diabetes (Nolte Fong and Moore, 2018). As argued by Taşdemir and Aksoy (2020), limiting increased calories intake supports lowering or control of weight gain in renal transplant patients. The choice of intake of raw vegetables and fruits, lean meat, whole grains, non-fat dairy products and others contributes to low calories intake in renal transplant patients which makes them avoid increased weight gain.
In contrast, the study by Bunde et al. (2021) mentions that physical activity is the key lifestyle change that allows lowering the weight gain and type-2 diabetes risk in renal transplant patients after the surgery. The physicians prefer inclusion of physical activity among renal transplant patients because it is considered to show a promising impact on controlling weight gain and diabetes in renal transplant patients. This is because physical activity helps the body to overcome stress caused by the treatment procedure due to supporting release of endorphins in the brain that are natural painkillers leading to improve sleep and calmness in patients (Taşdemir and Aksoy, 2020). Moreover, physical activity helps in the use of increased fats through food in the body resulting to avoids its deposition that could lead to weight gain in the patients (Takahashi et al., 2018). However, there are lack of research that have focussed on the perception of weight increase and type 2 diabetes in renal transplant patients in terms of physical activity.
In most of the existing studies analysed, the overview regarding diabetes and weight gain in renal transplant are gathered from researchers. However, the way renal transplant patients view the problem and consider it to be resolved are not focused. The studies also failed to provide insight regarding the preference and hindrance faced by renal transplant patients which could influence their acceptance of executing physical activity for managing risk of weight gain and diabetes. The present study is also being developed, and it will give physiotherapists with information about the patient perspective on physical activity recommendations for renal transplant patients. This is because patients with kidney transplants frequently report avoiding physical therapy or physiotherapy help for physical exercise out of concern of graft damage and other transplant related psychosocial issues (Sokunbi, 2017). As a result, the current study is been designed to learn more about how renal transplant patients think about weight gain and diabetes type 2 diabetes in terms of physical activity.
2. RESEARCH AIM & OBJECTIVES
Research Aim
The primary goal of this qualitative study is to learn more about how renal transplant patients think about weight gain and type 2 diabetes in terms of physical activity.
Research Objectives
To identify how individuals with kidney transplants think about weight gain and type 2 diabetes in terms of physical activity.
To analyse the factors influencing preference of physical activity as important component for avoiding weight gain and type-2 diabetes in renal transplant patients
To evaluate the challenges experienced with physical activity in renal transplant patients to avoid weight gain and type-2 diabetes
To develop recommendations in resolving the challenges experienced with physical activity in renal transplant patients to avoid weight gain and type-2 diabetes
3. Research Design
The qualitative phenomenological research design will be used in executing the study. This is because qualitative design allows understanding the attitudes and feelings of the target population which assist in gathering enhanced perception of participants regarding a given topic (Burns et al., 2017). Phenomenology is a type of qualitative research that examines a person’s living experiences in the world. In the current study, perception of renal transplant patients regrading physical activity as significant intervention for diabetes and weight loss is been focussed and the use of qualitative information will help in gathering the required information for accomplishing the study. The qualitative research design will also be used because it assists in saving money by including lower number of participants for the study. The reduce participants required less cost of management to gather information from them (Andersen et al., 2019). The qualitative research design will be used because it allows creativity being a driving force in accomplishing any study. It is evident as the design helps the participants answer the questions in a way that are real and not mentioned to please the researchers leading the results mentioned to be more accurate (Burns et al., 2017).
4. Setting
The study will be executed in the Guy’s and St Thomas NHS Foundation Trust in the UK. This is because it is the most frequent place where most life-saving kidney transplants are done in the UK since 2019-2020. It is evident 241 kidney transplant patients are treated in the centre (guysandstthomas.nhs.uk, 2020). Since such huge number of patients are present in the centre, it will be easier to find potential participants required for the current study. In order to execute study of patients in the Trust, permission from the head is to be achieved by relating the person regarding the need and importance of the research topic.
5. Participants
5.1 Inclusion Criteria
The patients who will be included in the study are required to have received first-hand kidney transplant within one year since the date of the current study. This is because weight gain and type-2 diabetes risk are mostly prevalent in renal transplant patients within 1-2 year of their transplant out of continued post-medication and treatment (Klaassen et al., 2017). The participants who are considered to be at increased risk of developing type-2 diabetes and have three criteria such as below the age of 50 years, BMI is below 30 kg/m2 and has family history of type-2 diabetes will be included. The participants who are within 18-50 years of age, has signed the informed consent form, are either men or women, affiliated to medical care system and receiving immunosuppressive medications like mycophenolic acid, tacrolimus and others will be included in the study. The participants in whom the steroids and immunosuppressive medication are considered for cessation in the next 3 three months after the transplant will be included.
5.2 Exclusion Criteria
The participants who will be excluded from the study are those who have limited capacity in mentioning their views and thoughts, show lack of cooperation with the investigator, has no health insurance, are pregnant and breastfeeding, suffer from hepatic insufficiency, obese, patients with heart failure and inability to understand the cause for the study. This is because such patients who lead to create barrier in fair explanation of their perception in participation for physical activity after renal transplant. The patients who have any form of active infection, personal history of diabetes, infected with hepatitis C virus, history of pancreatitis, has angioedema and suffers from multi-organ failure will not be included in the study. This is because the additional health issues would hindered accessing fair view of the impact and perception of physical activity in renal transplant patients.
6. Outcomes
The semi-structured interview is referred to as the interview type which includes the interviewer asking for few pre-determined questions while the rest questions which are not planned beforehand are also asked in the session. This indicates that the process follows both the structured and unstructured interview styles in gathering information (Ralph et al., 2019). In this study, the semi-structured interview will be used for data collection purpose and presenting outcomes for the study. The semi-structured interview will be used as it allows enhanced flow of conversation between participants and the researchers compared to other interview sessions (Ralph et al., 2020). This is because in semi-structured interview all the questions are not previously prepared and based on the progression of the interview and data sharing by the participants. The researcher gradually frames questions and extend the conversation in a friendly way so that enhanced flow of interaction between the two can be achieved without creating any awkwardness due to previously set question which the participants are not familiar to answer.
The semi-structured interview will also be used in the study as it provides room for the researcher or interviewer to ask different nature of open-ended questions that are personally tailored to avoid general questionnaires. It provides opportunity to the interviews to present their opinion in creative way without being judged for drifting away from interview structure (Lorenz et al., 2019). The semi-structured interview is able to be tailored as per the skillset and experience of a person. It indicates that the interviewer is able to ask question based on the education and informative level of the participant. It opposes blank questioning that is used for everyone in structured interview (Nielsen et al., 2019). Therefore, the semi-structured interview process will be used in framing outcomes for the study. However, the issue with semi-structured interviews is that increased time is required for making conversations as such interviews are made based on the flow of the interaction that typically require more time than structured session (Tarabeih and Bokek‐Cohen, 2020). Thus, the requirement of time will be explained to the participants so that the individual can understand the amount of time required and ensure the person remain free during the session to avoid it from inappropriately concluding due to hindered presence of time.
The other issue with semi-structured interviews is that it may make the interviewer leave valuable questions to be asked to the participants if they get carried away in the conversations (Mercado-Martínez and Levin-Echeverri, 2017). Thus, for limiting the issue, the set of important question necessary to be asked will be framed and it will be ensured they are asked as well as replied properly by the participants within the interview session no matter how far the interview extends. The other problem with semi-structured interview is that they may be biased. This is because the semi-structured interview requires no need of asking similar question to the participants which create room for presence of irrelevant bias such as ageism, sexism and others (Mahdizadeh et al., 2020). In order to limit the barrier, any form of discriminatory belief is to be avoided while making conversation and ensure a similar pattern is follow in making interaction with each of the participants.
In this study, the reliability of the semi-structured interview will be ensured by making one-to-one correspondence between the interview questions been asked and their underlying competency regarding the topic. For this purpose, important question in relation to the topic will be framed ahead of the session so that the interviewer is prepared and appear competent during the procedure. The use of semi-structured interview in the study ensures high validity because the interviewees are allowed to mention in-depth explanation of their attitude and feelings regarding the questions asked rather than the information to be interpreted from the perception of the interviewers due to lack of time (Ford et al., 2018). Thus, use of semi-structured interview will ensure enhanced validity in the study because it provides opportunity for the patients to explain their in-depth opinion regarding physical activity for weight loss and diabetes after renal transplant.
7. Study Procedure
7.1 Recruitment of participant
The potential participants will be identified based on the inclusion and exclusion criteria by the researcher through physically examining their health records. Patient information will be collected through electronic communication medium. Formal request emails will be sent to the NHS consulting physician, requesting to provide with necessary patient details. In order to allow potential patients to publicly involved in the study, posters of the mentioned study and way along with need to be performed will be circulated in the Trust notice board. The potential participants who are willing to involve will show their interest in the project by replying to the email for participation and signing the consent form for the study. All the information regarding the study will be shared online through email with the participants with effective proof of authenticity that is sharing my university reference number. A minimum of 15 days will be provided to participants to finalise their action to take part in the study.
7.2 Consent
In order to gather consent from the potential participants, Patient Information Sheet and Consent Form will be provided to them through email to avoid in-person contact in the current Covid-19 situation. In the form, the email providing the consent form, personal number of the researcher will shared with mention of time when the participants can call and ask any queries to resolve any doubts in providing their consent. The capacity of the participants in providing their consent will be assessed on their level of understanding of the project, ability to provide response and making own decision without any influence. The participants will be explained in detail the process of the study and in any chance of coercion, they are to explained way their personal interest will be protected during the study to retain their privacy and confidentiality.
7.3 Withdrawal Criteria
The potential participants are allowed to withdraw from the study any time if they feel their confidentiality is compromised under any condition. Moreover, they are allowed to withdraw if they feel threatened from the interviewer and consider sharing information with the person is leading them to experience stress. The participants who have not fulfilled the inclusion criteria and abuses the interviewer will be automatically withdrawn from the study with explanation of the point due to which the participants are withdrawn.
8. Data Analysis and Handling
8.1 Data Collection
The Data Collection will be made by using Microsoft teams as the tool for the selected participants. The potential participants will be required to have or open Skype account to allow virtual face-to-face interview with permission previously provided to interviewers through consent form that the interview could be recorded. Skype is to be used because it allows video calling option and avoids requirement of physical presence in the interview environment (Cheng, 2017). In the current Covid-19 condition, the Skype will be used for interviewing so that social distancing with the patients are made which is rule as per the government guidelines. Moreover, it will also help in recording the verbatim and facial expression of the interviewees to execute in-depth analysis in framing well-develop outcome for the study. The attitude, feelings, experience and knowledge of the renal transplant patients regarding physical activity use to reduce risk of weight gain and type-2 diabetes are the data that will be gathered in the study. The verbatim and audio, as well as video recording of the interview, will be done.
8.2 Data Handling
The gathered data will be manually processed by the researcher by comprehending the verbal and emotional cues provided by the interviewees during the interview. The data transcription will be executed by the researcher and it will be rechecked by a third-party researcher to ensure the transcribed data are not different from the original response of the participants. The data will be stored in a separate folder for research in the personal computer that will be password protected. the password will be only known to the researcher responsible in executing the study. The data recording will be made at the beginning by anonymising the name of the interviewees in the form of interviewee 1 and so on. The researcher will be responsible in making data entry and ensuring quality and will be also responsible for analysing data.
8.3 Data Analysis
The data analysis will be executed by implementing thematic analysis method mentioned by Baurn and Clarke. This is because the thematic analysis allows qualitative data to be gathered for presentation in proper patterns and themes (Clarke and Braun, 2014). Thus, it will be used because the process will allow different ways of analysing the verbatim of the participants that area qualitative data to sort the data into different themes.
8.4 Record Keeping
The data gathered will be stored for 6 months after which it will be destroyed by deleting the content. The information will not be archived because it contains valuable opinion of the patients which if known may be used against them to cause harm or abuse towards them. Thus, for the safety of the participants, the data will be destroyed at the end of 6 months.
9. Regulatory Issues
9.1Ethics Approval
The researcher will seek ethical permission for the aforesaid study from MMU Ethics Online System (EthOS). The participants in the study will be NHS patients, the Health Research Authority will need to approve the study(HRA).
9.2 Health and Safety
There are no significant health risk identified that will be faced by the participants by involving in the study.
9.3 Conflicting Interest
In the study, no conflict of interest will be faced as researchers executing the study will be effectively following roles and responsibilities which meet the obligations required in executing the study. However, the competing role of recruiting own participants will be faced in the study. For this purpose, a gatekeeper will be allowed to support the researchers in selecting participants based on inclusion and exclusion criteria so that competing role is avoided and mutual decision towards effective selection of participant will be accomplished.
9.4 Confidentiality and Data Protection
The data controller and custodian in the study will be the researchers executing the study. They will have access to personal data during as well as after the project. The personal data will be stored in the personal computer with secured password only known to the researchers and limiting access to the content by allowing only approved individuals by researchers to view the data under their supervision. The researchers will be responsible for destruction of data at the end of the research. During transfer of data, it will be shared in anonymous way by avoiding mentioning the name of the participants and legal right to share the data is to be gathered from the participants through written consent.
10. Dissemination Policy
The data arising from the study will be owned by the researchers who have presented them. The authorship agreement for publication and final reporting will include initially presenting draft of the article for revision based on which the final version of the study will be developed to be reviewed for publication. In the study, no funding bodies will be involved and so there are no supporting bodies who would be acknowledged within the publications. However, the third-party researchers who are involved in analysing and authenticating the data interpretation of the researchers will be acknowledged in the study. The publication rights of the data from the study will remain with the researchers who will frame the study. There are no possible future plans to make the gathered data public due to confidentiality issues for the patients. However, the outcome in the study will be notified with sample copies to the participants which will be sent via email to help them understand the way their views are used from the interview in framing the study as well as their confidentiality is maintained.

11. Project timeline
The study will not proceed unless it has received ethical approval. MMU clearance and HRA approval might take up to 2 months, according to the researcher. The recruiting participants will take another 30 days. The interviews will take place over the course of 15 days. Over the next two weeks, the interviews will be transcribed. Following that, thematic data analysis and documentation will take 2 months to complete, allowing the researcher to continue working while the study is being conducted. This study is expected to take 6 months to complete.
Continue your journey with our comprehensive guide to Thio-Yne Click Hydrogels for Enhanced Control.
References
Andersen, M.H., Wahl, A.K., Engebretsen, E. and Urstad, K.H., 2019. Implementing a tailored education programme: renal transplant recipients’ experiences. Journal of renal care, 45(2), pp.111-119.
Boerner, B.P., Miles, C.D. and Shivaswamy, V., 2014. Efficacy and safety of sitagliptin for the treatment of new-onset diabetes after renal transplantation. International journal of endocrinology, 2014.pp.67-90.
Bunde, K., Gjesvold, D., Kattelmann, K.K., McCormack, L.A. and Vukovich, M.D., 2021. Increased Frequency of Nutritional Counseling Improves Weight Status and Lipids in Renal Transplant Recipients. Topics in Clinical Nutrition, 36(1), pp.3-12.
Burns, T., Fernandez, R. and Stephens, M., 2017. The experience of waiting for a kidney transplant: A qualitative study. Journal of renal care, 43(4), pp.247-255.
Cheng, F.K., 2017. Using email and skype interviews with marginalized participants. SAGE Publications Ltd.
Clarke, V. and Braun, V., 2014. Thematic analysis. In Encyclopedia of critical psychology (pp. 1947-1952). Springer, New York, NY.
Ford, J.D., Spinazzola, J., van der Kolk, B. and Grasso, D.J., 2018. Toward an empirically based developmental trauma disorder diagnosis for children: Factor structure, item characteristics, reliability, and validity of the developmental trauma disorder semi-structured interview. The Journal of clinical psychiatry, 79(5), pp.12-45.
Forte, C.C., Pedrollo, E.F., Nicoletto, B.B., Lopes, J.B., Manfro, R.C., Souza, G.C. and Leitão, C.B., 2020. Risk factors associated with weight gain after kidney transplantation: A cohort study. PLoS One, 15(12), p.e0243394.
Gaiffe, E., Crepin, T., Bamoulid, J., Courivaud, C., Büchler, M., Cassuto, E., Albano, L., Chemouny, J.M., Choukroun, G., Hazzan, M. and Kessler, L., 2019. PRODIG (Prevention of new onset diabetes after transplantation by a short term treatment of Vildagliptin in the early renal post-transplant period) study: study protocol for a randomized controlled study. Trials, 20(1), pp.1-11.
guysandstthomas.nhs.uk 2020, Guy's surgeons perform most kidney transplants in the UK, Available at:
Hollis, E., Shehata, M., Khalifa, F., Abou El-Ghar, M., El-Diasty, T. and El-Baz, A., 2017. Towards non-invasive diagnostic techniques for early detection of acute renal transplant rejection: A review. The Egyptian Journal of Radiology and Nuclear Medicine, 48(1), pp.257-269.
Issa, N., Sánchez, O.A., Kukla, A., Riad, S.M., Berglund, D.M., Ibrahim, H.N. and Matas, A.J., 2018. Weight gain after kidney donation: Association with increased risks of type 2 diabetes and hypertension. Clinical transplantation, 32(9), p.e13360.
Klaassen, G., Zelle, D.M., Navis, G.J., Dijkema, D., Bemelman, F.J., Bakker, S.J. and Corpeleijn, E., 2017. Lifestyle intervention to improve quality of life and prevent weight gain after renal transplantation: Design of the Active Care after Transplantation (ACT) randomized controlled trial. BMC nephrology, 18(1), pp.1-13.
Kluch, M., Kurnatowska, I., Matera, K., Łokieć, K., Puzio, T., Czkwianianc, E. and Grzelak, P., 2020. Nutrition Trends in Patients Over the Long Term After Kidney Transplantation. In Transplantation proceedings. 52(8). pp. 2357-2362.
Liaveri, P.G., Dikeos, D., Ilias, I., Lygkoni, E.P., Boletis, I.N., Skalioti, C. and Paparrigopoulos, T., 2017. Quality of sleep in renal transplant recipients and patients on hemodialysis. Journal of psychosomatic research, 93, pp.96-101.
Lorenz, E.C., Egginton, J.S., Stegall, M.D., Cheville, A.L., Heilman, R.L., Nair, S.S., Mai, M.L. and Eton, D.T., 2019. Patient experience after kidney transplant: a conceptual framework of treatment burden. Journal of patient-reported outcomes, 3(1), pp.1-9.
Mahdizadeh, A., Oskouie, F., Khanjari, S. and Parvizy, S., 2020. The need for renovating patient education in kidney transplantation: A qualitative study. Journal of Education and Health Promotion, 9.pp.67-89.
Mercado-Martínez, F.J. and Levin-Echeverri, R., 2017. Care for chronic kidney disease in Uruguay: the perspective of kidney transplant patients. Cadernos de saude publica, 33(10), pp.e00160416-e00160416.
Nie, H., Wang, W., Zhao, Y., Zhang, X., Xiao, Y., Zeng, Q., Zhang, C. and Zhang, L., 2019. New-Onset Diabetes After Renal Transplantation (NODAT): Is It a Risk Factor for Renal Cell Carcinoma or Renal Failure?. Annals of transplantation, 24, p.62.
Nielsen, C., Clemensen, J., Bistrup, C. and Agerskov, H., 2019. Balancing everyday life—Patients’ experiences before, during and four months after kidney transplantation. Nursing open, 6(2), pp.443-452.
Nolte Fong, J.V. and Moore, L.W., 2018. Nutrition trends in kidney transplant recipients: the importance of dietary monitoring and need for evidence-based recommendations. Frontiers in medicine, 5, p.302.
Nolte Fong, J.V. and Moore, L.W., 2018. Nutrition trends in kidney transplant recipients: the importance of dietary monitoring and need for evidence-based recommendations. Frontiers in medicine, 5, p.302.
Orazio, L., Chapman, J., Isbel, N.M. and Campbell, K.L., 2014. Nutrition care for renal transplant recipients: an evaluation of service delivery and outcomes. Journal of renal care, 40(2), pp.99-106.
Patel, S., Gohel, K. and Patel, B., 2016. Incidences and risk factor for new onset diabetes after transplantation in live donor kidney transplantation: a prospective single centre study. Int J Pharm Pharm Sci, 8(2), pp.230-3.
Ralph, A.F., Butow, P., Craig, J.C., Wong, G., Chadban, S.J., Luxton, G., Gutman, T., Hanson, C.S., Ju, A. and Tong, A., 2019. Living kidney donor and recipient perspectives on their relationship: longitudinal semi-structured interviews. BMJ open, 9(4), p.e026629.
Ralph, A.F., Chadban, S.J., Butow, P., Craig, J.C., Kanellis, J., Wong, G., Logeman, C. and Tong, A., 2020. The experiences and impact of being deemed ineligible for living kidney donation: semi‐structured interview study. Nephrology, 25(4), pp.339-350.
Rysz, J., Franczyk, B., Radek, M., Ciałkowska-Rysz, A. and Gluba-Brzózka, A., 2021. Diabetes and cardiovascular risk in renal transplant patients. International Journal of Molecular Sciences, 22(7), p.3422.
Sabbatini, M., Ferreri, L., Pisani, A., Capuano, I., Morgillo, M., Memoli, A., Riccio, E. and Guida, B., 2019. Nutritional management in renal transplant recipients: A transplant team opportunity to improve graft survival. Nutrition, Metabolism and Cardiovascular Diseases, 29(4), pp.319-324.
Sokunbi, G., 2017. Exercise and Rehabilitation: Needs for Kidney transplantation. J Physiother Res, 1(1), pp.1-2.
Takahashi, A., Hu, S.L. and Bostom, A., 2018. Physical activity in kidney transplant recipients: a review. American Journal of Kidney Diseases, 72(3), pp.433-443.
Tarabeih, M. and Bokek‐Cohen, Y.A., 2020. Between health and death: The intense emotional pain experienced by transplant nurses. Nursing inquiry, 27(2), p.e12335.
Taşdemir, D. and Aksoy, N., 2020. Weight Gain, Energy Intake, Energy Expenditure, and Immunosuppressive Therapy in Kidney Transplant Recipients. Progress in Transplantation, 30(4), pp.322-328.
Yepes-Calderón, M., Sotomayor, C.G., Gomes-Neto, A.W., Gans, R.O., Berger, S.P., Rimbach, G., Esatbeyoglu, T., Rodrigo, R., Geleijnse, J.M., Navis, G.J. and Bakker, S.J., 2019. Plasma malondialdehyde and risk of new-onset diabetes after transplantation in renal transplant recipients: a prospective cohort study. Journal of clinical medicine, 8(4), p.453.
Appendix
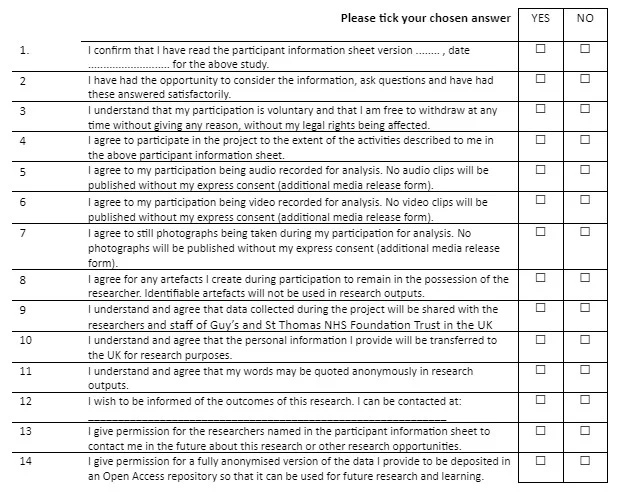
CONSENT FORM
Title of Project: Perception of Renal transplants patients towards the importance of physical activity in reducing the prevalence of weight gain and Type 2 diabetes
Participant Identification Number:

Patient Information Sheet
Perception of Renal transplants patients towards the importance of physical activity in reducing the prevalence of weight gain and Type 2 diabetes
1. Invitation to research
I/We would like to invite you to take part in the project which focusses on identifying ways to reduce prevalence of weight gain and Type 2 diabetes in patients who have suffered renal transplant in the last year. My name is ……… and I am the key author of the research project is Perception of Renal transplants patients towards the importance of physical activity in reducing the prevalence of weight gain and Type 2 diabetes. The project is developed by as a student researcher from the University and it is self-funded.
2. Why have I been invited?
You are being invited because you have experienced renal transplant in the last year and out of continued post-medication and treatment, above 50 years of with BMI above 30 kg/m2 and has family history of type-2 diabetes. The features make you more prone to type-2 diabeties development and weight gain which is adverse for your and health and thus effective pf physical activity is to be performed to ensure your good health and gather data to be included in research.
3. Do I have to take part?
It is up to you to decide. We will describe the study and go through the information sheet, which we will give to you. We will then ask you to sign a consent form to show you agreed to take part. You are free to withdraw at any time, without giving a reason.
4. What will I be asked to do?
You will be asked regarding your health history and course of medical intervention and others. No personal information relating to relationships and others are to be asked.
5. Are there any risks if I participate?
No, there are no identified risk which you may suffer.
6. Are there any advantages if I participate?
Yes. In case you participate, you will be able to enjoy free physiological assistance from physiotherapist and others to support you to be physical activity that would be effective for your health improvement.
7. What will happen with the data I provide?
When you agree to participate in this research, we will collect from you personally-identifiable information.
The Manchester Metropolitan University (‘the University’) is the Data Controller in respect of this research and any personal data that you provide as a research participant.
The University is registered with the Information Commissioner’s Office (ICO), and manages personal data in accordance with the General Data Protection Regulation (GDPR) and the University’s Data Protection Policy.
We collect personal data as part of this research (such as name, telephone numbers or age). As a public authority acting in the public interest we rely upon the ‘public task’ lawful basis. When we collect special category data (such as medical information or ethnicity) we rely upon the research and archiving purposes in the public interest lawful basis.
Your rights to access, change or move your information are limited, as we need to manage your information in specific ways in order for the research to be reliable and accurate. If you withdraw from the study, we will keep the information about you that we have already obtained.
We will not share your personal data collected in this form with any third parties.
If your data is shared this will be under the terms of a Research Collaboration Agreement which defines use, and agrees confidentiality and information security provisions. It is the University’s policy to only publish anonymised data unless you have given your explicit written consent to be identified in the research. The University never sells personal data to third parties.
We will only retain your personal data for 6 months as is necessary to achieve the research purpose and later it would be destroyed. The data would be stored anonymously, and your confidentiality would be respected in all context .
For further information about use of your personal data and your data protection rights please see the University’s Data Protection Pages.
What will happen to the results of the research study?
The results will be used in executing the current study.
Who do I contact if I have concerns about this study or I wish to complain?
If you have any concerns regarding the personal data collected from you, our Data Protection Officer can be contacted using the legal@mmu.ac.uk e-mail address, by calling 0161 247 3331 or in writing to: Data Protection Officer, Legal Services, All Saints Building, Manchester Metropolitan University, Manchester, M15 6BH. You also have a right to lodge a complaint in respect of the processing of your personal data with the Information Commissioner’s Office as the supervisory authority. Please see: https://ico.org.uk/global/contact-us/
THANK YOU FOR CONSIDERING PARTICIPATING IN THIS PROJECT
Research Insurance Checklist
Overview
Manchester Metropolitan University holds insurance policies to cover claims for negligence arising from the conduct of the institution’s normal business. This includes research undertaken by undergraduate and postgraduate students as part of their academic qualification as well as research carried out by staff.
If you are an undergraduate student, postgraduate student or staff researcher at the institution, you must complete all relevant sections of the checklist on the following pages to identify whether your application requires referral to the university’s Insurance Officer.
Completing and submitting the checklist will ensure that your research study has appropriate insurance cover in place before it begins. Please submit your completed Research Insurance Checklist along with your Ethics Checklist and/or Application for Ethical Approval to your Faculty Research Officer.
Referral to the Insurance Officer
If your research falls into any of the categories listed in Section 2 and/or Section 3 of the checklist, the Faculty Research Officer will send the following information to the Insurance Officer at insurance1@mmu.ac.uk:
Insurance Checklist,
Ethics Checklist and/or Application for Ethical Approval Form,
Participant Information Sheet(s) (if applicable),
Participant Consent Form(s) (if applicable),
Risk Assessment,
The Insurance Officer will liaise with the insurers to gain approval. Please note some types of research may require additional insurance, which may incur an additional cost to the Faculty.
Research studies must not commence until insurance and all other relevant authorisations and/or approvals are given.
Travel Insurance
Manchester Metropolitan University has a policy to provide worldwide travel insurance for members of staff and students travelling in connection with their course or on an approved University trip. This includes travel undertaken in connection with undertaking a research study. You must complete the online travel insurance form to register for travel insurance and should do this at least two weeks before your departure date.
Please visit the Financial and Legal webpage for details.
High Risk Countries
Please visit the Red24 website to identify whether the overall rating for the country you are travelling to is ‘High Risk’ or more severe. Please contact your Faculty Research Officer for guidance on accessing the relevant information on the website.

Lead Investigator Name Click here to enter text.
(Title/Forename/Surname)
Contact Email Address Click here to enter text.
Full Title of the Research Click here to enter text.

Physically invasive techniques?
This refers to any test in which the skin of the participant is broken or an implement is inserted into any opening of the human body (e.g. eyes, ears, nose, mouth, lungs, stomach, rectum, vagina and urethra) or involves the taking of body samples such as saliva, hair, urine, faeces, sputum, skin, nails, or taking biopsies of any form for any purpose, or any form of scanning such as DEXA scans, Ultrasound scans, MRI, fMRI, CT, or PET scanning.
Ingestion of food stuffs or drugs?
This refers to the consumption of any substance which may impact on psychological or physical state. Substances may include but are not limited to food, beverages or drugs.
Physical testing?
This refers to any test in which a participant must perform an action resulting in the use of any muscle of the body and/or involves the use of scanning procedures, eye-trackers, mounted body cameras, sensors or electrodes, or the taking of swabs from any cavity of the body, respiratory challenge testing or recording of peak flows, EEG, ECG, Exercise ECG, Treadmill work.
Psychological intervention?
This refers to any test which purposely alters the mood of the participant or involves administering personality inventories, or any other form of psychological test.
I confirm that my research does not fall into any of the above categories (please go straight to Section 3)

Pregnant persons as participants with procedures other than blood samples being taken from them?
Working with Hepatitis, Human T-Cell Lymphotropic Virus Type iii (HTLV iii), or Lymphadenopathy Associated Virus (LAV) or the mutants, derivatives or variations thereof or Acquired Immune Deficiency Syndrome (AIDS) or any syndrome or condition of a similar kind?
Working with Transmissible Spongiform Encephalopathy (TSE), Creutzfeldt-Jakob
Disease (CJD), variant Creutzfeldt-Jakob Disease (vCJD) or new variant Creutzfeldt-Jakob Disease (nvCJD)?
Working in hazardous areas or high risk countries? Please refer to the ‘High Risk
Countries’ guidance on Page 1 of this form.
Working with hazardous substances outside of a controlled environment?
Working with persons with a history of violence, substance abuse or a criminal
record?
I confirm that my research does not fall into any of the above categories
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



