Overview of the Medicines Act 1968
Medicines Act 1968 (Purpose, administration, enforcement);
Purpose
The medicine Act of 1968 is the UK parliamentary Act that governs the control of medicine for human, and veterinary use, which consists of manufacturing and supply of medicines. The Act states three categories of medicines: prescribes only medicine (POM), withiout prescription and General sale list (GSL) (NICE guidelines, 2016) which can be sale in shops without prescription, and pharmacy medicine (P) available for pharmicists. The Act Conrol supply of the drugs covers but doest define what consititute to an offence.
Administration
All medicines administered or issued to patients within NHS GG&C are procured by and distributed through pharmacies (either hospital or community), or via approved homecare suppliers, under local SOPs. Medicines are ordered, prescribed, administered or supplied to patients only by suitably competent practitioners, within a legal framework, who can exercise professional accountability and judgment in the best interests of their patients. This includes persons operating within a Patient Group Direction, (PGD), or functioning as non-medical prescribers. Self-administration schemes, e.g. patient-controlled analgesia (PCA) must incorporate a formal patient assessment which should be documented. There are systems for the transport of medicines that ensure their security, quality, and integrity, and maintain the health and safety of the patient, staff and the public.
Enforcement
Patients own drugs (PODs), including CDs, may be used during the hospital stay, where they assessed as suitable for use, the patient consents to their use, and suitable local SOPs are in place (NICE guidelines, 2016). All medicines will be stored securely in pharmacy and at the ward, theatre or department level to maintain their quality and security in suitable cupboards, refrigerators, and freezers as appropriate. Medicines ordered, prescribed and administration recorded on approved stationery, which must be stored securely to prevent fraudulent use (NICE guidelines, 2016). Electronic prescription systems can be employed provided there are procedures in place to restrict access to terminals and passcodes and ensure a full audit trail maintained. Medicines are prescribed in line with agreed formularies, or have the required level of approval for use if a patient is admitted or commenced on a non-formulary medication Unlicensed medicines, except for drugs being used for research or in clinical trials, are used only when no suitable licensed medicinal product is available. Disposal of medicines complies with legal requirements and health and safety regulations. There must be a complete audit trail available from purchase and receipt by pharmacy or approval of the use of PODs, to their administration to private in-patients, supply at the point of discharge, return to the pharmacy or their destruction at ward/theatre/ department level.
Licensing of Medicines
Licencesing and certification relating to medicinal products is found in part two of the medicine Act 1968. All authorisations for medicinal products for human use and licences for manufacturers and wholesalers of such products across the UK are issued by the MHRA. Through a Memorandum of Understanding, the Department has agreed with MHRA that while inspection and enforcement for these particular matters remains the Department’s responsibility (NICE guidelines, 2016), operationally the MHRA Inspectorate should be the lead authority for related inspection arrangements across the UK.
Looking for further insights on Influencer Marketing? Click here.
Manufactures and Wholesale Dealers License.
The Medicines for Human Use (Manufacturing, Wholesale Dealing and Miscellaneous Amendments) Regulations 2005 (SI2005/2789). The whole of the Regulations was revoked and consolidated by the Human Medicine Regulations 2012 which came into force on 14 August 2012. These Regulations implemented certain provisions of Directive 2004/27/EC of the European Parliament and of the Council ('the 2004 Directive') (Skaczkowski, et al 2016). amending Directive 2001/83/EC on the Community code for medicinal products for human use ('the 2001 Directive'), made changes to certain existing provisions which implemented Directive 2001/83/EC and made consequential amendments to various enactments. The Regulations implemented the requirements of the 2004 Directive insofar as they related to the manufacture, assembly, importation and wholesale distribution of medicinal products to which those Directives apply ('relevant medicinal products'), and, as respects relevant medicinal products, replaced the Medicines (Standard Provisions for Licences and Certificates) Regulations 1971, as amended, which implemented the requirements of the 2001 Directive as respects those matters. Regulation 9 imposed a requirement that wholesale dealers dealt only with specified persons.
‘Specials’
Most drugs prescribed for patients as part of their NHS treatment are licensed medicinal products. However, there are rare occasions when a patient needs a formulation or strength that is not available as a licensed form, or needs to avoid ingredients that provoke allergy (Steele, Adcock, Steel, 2016). To address such needs, many specially prepared products are produced by specialist manufacturing units. These so-called ‘specials’, unlike licensed medicines, are not assessed for safety or efficacy by a regulatory body. Prescribers are often unaware of the costs of specials. Here we explore some of the issues associated with specials in primary care and whether their use represents good value for money.
Parallel Imports
To market a parallel-traded product, an importer needs an abbreviated marketing authorisation (PL[PI]) from the Medicines and Healthcare products Regulatory Agency (MHRA). Regulation 4A of the Medicines (Labelling) Amendment Regulations 1992 stipulates that all medicinal products in the UK must be labelled in the English language only (NICE guidelines, 2016), or in English plus one or more other languages, provided the same particulars appear in all the languages. For EU imports labelled in a language other than English, the importer must relabel the medicines to comply with the regulations, by repackaging the medicines or overlabelling the existing packaging.

Counterfeit medicines
Counterfeiting is a major problem abroad and a growing concern for UK authorities. The prevalence of counterfeit medicines in the legitimate supply chain is uncertain but few have been identified. The internet is believed to be a major source of fake medicines but the scale of the problem in the UK is unclear. To date there is little evidence of harm to NHS patients but the surveillance systems in place seem weak given the scale of medicines distribution (NICE guidelines, 2016). Current strategies to tackle the problem include increasing awareness among the public and healthcare professionals, product tracking and developing secure lines of supply.
Good distribution Practice
According to Information from European Instituions, bodies, officers and agencies, guidelines of 2013 on good distribution practices of medicinal product for human use highlighted in six chapters. Chapter one, contains quality management practices which includes, quality distribution system, outsources activities, review and monitor of distribution chains and quality risks management. Chapter two, dwels on personnels in regards to reposnible person, training, hygiene. Chapter three deals with premise, equipments (Nursing and Midwifery Council, 2014). Chapter four; tackles operations in respect to the suppliers and, customers qualifications, receipt, storage, destruction of obsolete goods, picking , supply and export to third party. Finally, the complains, returns, suspected falsified medicinal product and medicinal product records.
Records
The three pieces of legislation governing access to patient health records are The Data Protection Act 1998, The Access to Health Records Act 1990 and The Medical Reports Act 1998. The first governs the rights of living individuals and authorised persons, the second governs access to deceased patient’s records, and the third outlines the rights of individuals to access reports relating to themselves provided by medical practitioners for employment or insurance purposes. The release of any record is subject to consultation with the health professional. Hospital records are retained for a minimum of eight years, whilst GP records are retained for a minimum of 10 years. There is a charge for access or viewing the records with the Government stating that patients should be given access to their health records within 21 days following a request
Classification of Medicines: GSL; P; POMs
Medicines which are Pharmacy medicines do not require a prescription but can only be supplied by a registered pharmacy or a veterinary surgeon. POM drugs are available on prescription only, as issued by a veterinary surgeon, and can only be supplied for animals that are "under the care" of the prescribing veterinary surgeon (NICE guidelines, 2016). Medicines on the General Sale List may be obtained without a prescription and some may be sold by e.g. pet shops and supermarkets (for example some anthelmintics).
Packageging size restriction
Implements obligations found in Title 5 of Directive 2001/83/EC by consolidating provisions previously in Part V of the Medicines Act 1968, the Medicines for Human Use (Marketing Authorisations Etc) Regulations 1994 (SI 1994/3144), and the Medicines (Child Safety) Regulations 2003 (SI 2003/2317) (NICE guidelines, 2016). It sets out the information that must appear on packaging and in leaflets, and contains specific rules for Braille, radionuclides, and homeopathic and herbal medicinal products.
Retail Sale
Current legislation allows health professionals and others to sell, supply and administer medicines by way of exemptions from the usual Medicines Act restriction. Exemptions that are obsolete or no longer relevant will be removed. A provision that allows pharmacists to sell or supply amyl nitrite will be removed. (Amyl nitrite used to be administered in cases of cyanide poisoning but it is no longer recommended as an antidote by the Health and Safety Executive.) It is also proposed that those requiring water for injections, currently classed as a prescription only medicine, for purposes other than parenteral administration (eg, to inflate balloons in catheters), will be able to obtain it without a prescription. The MHRA also wants to allow pharmacists to sell or supply water for injections as a diluent where no diluent has been specified by the prescriber. This will avoid unnecessary delay in administering medicines when a dry powder for injection has been prescribed without the necessary diluents.
Supervision of sale and supply
POMs Prescriptions and supply
A Prescription Only Medicine may be sold or supplied by retail only in accordance with a prescription given by an appropriate practitioner. United Kingdom registered doctors and dentists are appropriate practitioners for all Prescription Only Medicines, as are doctors and dentists registered in an EEA country or Switzerland (NICE guidelines, 2016). EEA or Swiss healthcare professionals are not permitted to issue prescriptions for Schedule 1-5 Controlled Drugs and medicines that do not have a UK marketing authorisation.
Types of Precribers
Under UK law, only “appropriate practitioners” can prescribe medicine. A prescriber is a healthcare professional who can write a prescription. It applies across private and NHS prescriptions. Appopriate practitioner falls under two categories: Either as an independent prescriber, someone able to prescribe medicines under their own initiatives or a supplementary prescriber; someone able to prescribe medicines in accordance with a pre-agreed care plan that’s been drawn up between a doctor and their patient. Independent prescribers includes; doctors, dentists, nurse indepent prescribers, pharmacists independent prescribers, optometrists independent prescribers, podiatrists, pysiotherapiests and therapeutic radiologists. The supplementary prescribers are nurses/midwives, pharmacists, diagnostic radiographers, therapeutic radiographer, optometrists and dietitians.
Electronic Prescribing
NHS hospitals in England are expected to be paperless by 2020, as set out in a comprehensive framework published by the National Information Board. The use of hospital electronic prescribing (EP) systems is therefore likely to increase rapidly in the near future.
Prescribing conventions
Depending on the expression context, prescribing conventions is a standard prescribing of drugs in every conditions required by NICE guidelines or clinical knowledge summaries. It was initially known as prodigy.
Prescrption types
There are three types of Prescription Drugs. All drugs fall into three general categories because of the usual effects they create. It is categorized into depressants, opioids and morphine derivative and stimulants. Depressant Drugs: Depressants are usually used to cause the body to feel more relaxed (NICE guidelines, 2016). They are used for sleep medications when someone is having trouble relaxing enough to fall asleep at night. Most popular depressant named after street Ambien, Valium, Roofies, Barbs, etc. Depressants come in both legal forms and illegal forms. Depressants can cause drowsiness, which can make it dangerous. Opioids and Morphine Derivates: Opioids are used most commonly for pain relief. They often derive from the poppy plant. These drugs are taken orally or through a needle. They are useful when one is in extreme pain, but they can be fatal when misused. Opioids are addictives; they include Codeine, Tylenol, schoolboy. Prescription stimulants; the third type of prescription. Stimulants are different from the two descriptions above. They increase the feeling of mental alertness.
Charges and Exemptions.
There have been suggestions from some stakeholders that additional changes could be introduced to relieve pressure on the NHS budget. However, the government has repeatedly stated that NHS GP appointments are a free to charge. For example in October 2014, the Chief Executive of the NHS Confederation, Rob Webster, suggested the patients may have to cover their hotel costs for bed and board. The exemption categories are age 16 years of age, full-time students aged 16, 17, 18. Another exemption is people over 60 years and maternity: prescription pre-payment certificate, war pension exemption. Pension credit and prisoners are among the exception.
Patient Group Direction
Patient Groups Direction is a written instruction for the supply or administration of medicines to groups of patients who may individually identify before treatment. It can acts as a direction to a nurse to supply and administer prescriptions only on medication to patients using their assessment.
Emergency Supply
The emergencies supply provisions of Regulations 8 of the Medical Products Regulation amendments 2003 permits pharmacists, in emergency circumstances, to provide specific prescription only carried out at the requests of a patient or the application of the prescriber. When supplying a medicine under these provisions, the pharmacists must be satisfied that there is an immediate need for medication provided
Provision of servives in pandemic or other national emergency
The UK government in its 2010 White Paper “Equity and excellence: Liberating the NHS” announced a strategy on how it will “create a more responsive, patient-centred NHS which achieves outcomes that are among the best in the world” (Steve, 2013). The section presents an overview of the UK healthcare system as it currently stands, with emphasis on Predictive, Preventive and Personalised Medicine elements.
Forged Prescrption
Forging or altering a prescription is a serious offense that is treated like any other drug offense. The government takes prescription drug fraud very seriously and the consequences can be serious for anyone involved. Anyone can be charged with prescription drug fraud, including: doctors, doctors assistants, nurses and private individuals. These crimes are difficult for law enforcement to investigate because there are many privacy laws surrounding medical situations.
Record
The NHS Constitution outlines patients’ rights to privacy, confidentiality, security of their medical records, and to be informed about how their information is used. The Health and Social Care (Safety and Quality) Act 2015 introduced a duty for health and social care commissioners and providers to share patient information where they consider that the disclosure is likely to facilitate the care provided to the individual and is in their best interest. Patient information must be securely safeguarded, although individuals also expect that relevant health information is shared amongst their care team.
Labelling and packaging regulations: PIL; Child resistant closures; fluted bottles.
For substances and mixtures contained in packaging that is small (typically less than 125ml) or is otherwise difficult to label, CLP provides for exemptions from the regular labelling requirements (Steve, 2013). These exemptions allow the supplier to omit the hazard and/or precautionary statements or the pictograms from the label elements normally required under CLP in case the substance or mixture is classified for the hazards listed in section 1.5 of Annex I to CLP
Promotion and sales of medicine
The legal requirements to advertise and promote your medicine including the Blue Guide which interprets the law in more detail. Proprietary Association of Great Britain (PAGB) reviews all of their members’ advertising for over-the-counter medicines to the public against their codes of practice. The Committee of Advertising Practice also offers a free advice service for non-broadcast advertisements directed at the public. Material must comply with the UK Code of Non-Broadcast Advertising, Sales Promotion and Marketing. Also note that the MHRA may request to see your advertising before it is published to ensure that it complies with regulations.
Homeopathic medicines and herbal remedies: Licences and classifications
From the early 1970s a herbal medicine making a medicinal claim on the label required a Product Licence. Acceptable products already on the market were granted Product Licences of Right (PLRs) with minimum formality at the time (Opioid dependence, 2015) , but thereby became subject to general regulatory requirements for medicines including, for example, the premises in which they could be manufactured. The European Community Review of Medicines during the 1980s required such products to be reviewed for quality, safety and efficacy by 1990. Those that satisfied the requirements were granted a Product Licence. Herbal medicines: Apply for a traditional herbal registration, herbal medicines granted a traditional herbal registration and list of banned or restricted herbal ingredients for medicinal use. Homeopathic medicine or remedy register homeopathic.
Poison rules (schedules)
The recent introduction of the Deregulation Act has resulted in substantial changes to the Poisons Act 1972, affecting the sale of poisons in the UK.
Labeling and storage
Medical Device & Diagnostic Industry Magazine MDDI Article Index An MD&DI May 1999 Column The five-year transition period has passed since the implementation of the Medical Devices Directive, and products must meet its essential requirements before they can earn the CE mark and be sold in the EU market. Labeling techniques involves bar codes and regulatory lebaling trends. The local language labeling require less space with more symbols, overall streamline of documentation and more bar codes usages (HIBC, UCC and 3-D). Industry movement towards electronic labeling contains lebaling documentation stored in CD ROMs or DVD, or labeling documentation distributed through company websites.
Inspection and enforcement
The Department of Health, Social Services and Public Safety has responsibility for inspection and enforcement under all medicines related legislation in Northern Ireland. This is assumed by the Medicines Regulatory Group (MRG) within the Department (Dowell et al. 2015). The governing medicines-related legislation under which MRG acts includes the Human Medicines Regulations 2012, the Medicines Act 1968 and the Misuse of Drugs Act 1971 (together with a raft of attendant subordinate legislation), the Pharmacy (Northern Ireland) Order 1976 and the Poisons (Northern Ireland) Order 1976.
Schedule 1 poisons: sale and supply ;records; signed order exemption from requirements
Schedule 1 to the 2001 regulations covers drugs that have no therapeutic value and are usually used mainly in research under a Home Office licence. Examples include cannabis, MDMA (‘ecstasy’) and lysergamide.
Classification
The Misuse of Drugs Act 1971 lists controlled drugs in 1 of 3 classes – A, B and C. Class A drugs are considered the most harmful. Each class attracts different levels of penalties for a range of unlawful activities including the possession, supply and production of a controlled drug (Steve, 2013). In 2012, the Sentencing Council issued a definitive guideline for drug offences. The Home Office publishes a list of controlled drugs. It lists only the most commonly encountered drugs and is not exhaustive.
Cascade Process
Under UK law, the prescribing cascade for veterinary medicines (VMGN 13). Veterinary medicines guidance note for vets about how to imply the prescribing cascade for cascade for veterinary medicine. The Cascade is a risk based decision tree that allows you to use your clinical judgement to treat an animal under your care by deciding which product to use when there is no authorised veterinary medicine available in the UK (Steve, 2013). As part of the Royal College of Veterinary Surgeons (RCVS) Code of Professional Conduct for Veterinary Surgeons, you must obtain the owner’s consent for their animal to be treated under the Cascade.
Labeling dispensed medicines
The information that must be included on the label for products used under the Cascade is listed in the Veterinary Medicines Regulations (VMR). If all or part of the information cannot be included on the label, you may include it on a separate sheet. It is the responsibility of the person supplying the medicine to ensure it is appropriately labelled.
Records
As well as the normal record keeping requirements there are specific requirements for vets who administer or supply medicines under the Cascade. These must be kept for 5 years and made available upon request from a duly authorised person. The records that must be retained are listed in the VMR.
Drug Dependency
Dependence on a substance means that you need that particular substance to function normally. Drug dependence can result from prescribed drugs, recreational drugs or medicines available over the counter. Drug dependence is a treatable medical condition (Drug misuse and dependence, 2017) There are a number of medicines that your doctor may prescribe to help with drug dependency. The type of medicine prescribed depends on the drug you are dependent on.
Tolerance
A person may develop tolerance to a drug when the drug is used repeatedly. For instance, when morphine or alcohol is used for a long time, larger and larger doses must be taken to produce the same effect (Gianaroli, et al. 2016). Usually, tolerance develops because metabolism of the drug speeds up (often because the liver enzymes involved in metabolizing drugs become more active) and because the number of sites (cell receptors) that the drug attaches to or the strength of the bond (affinity) between the receptor and drug decreases.
Supply of paraphernalia to misuses
Parapheenalia supply has been menace in UK legal aspects since needle exchange inceptions. There are two main legal issues: One is the misuse of Drugs Act of 1971 has a subsection 9a (Cavanagh, 2018) which at that time made it offence for a person to supply needle or syrienge in cases where suppliers believe it has been used. Two: the Medicine Acts, water for injections was prescription only medicine making it illegal to supplie needle exchange.
Supervision and consumption
Supervised consumption services (SCS) are legally sanctioned facilities designed to reduce the health and public order issues often associated with public injection. They are also called safer injection facilities (SIFs) or overdose prevention centers (Dowell et al. 2015). These facilities provide a space for people to consume pre-obtained drugs in controlled settings, under the supervision of trained staff, and with access to sterile injecting equipment. Participants can also receive health care, counseling, and referrals to health and social services, including drug treatment.
Needle and syringe exchange scheme
The Needle and Syringe Exchange Scheme is a free, confidential service for people who inject drugs. It is designed to reduce the spread of blood borne viruses by providing free, sterile injecting equipment and by disposing of used equipment safely (Braileanu, Tavella, & Rousseau 2018). The PDF explains how the scheme works and provides contact details for all the participating pharmacies, and the local outreach teams
ADDITIONAL REQUIREMENTS FOR USE OF CDs IN HOSPITALS
The transfer of controlled drugs for use in the hospitals must be recorded in the Medicines Transfer Record Book and also be fully documented in the CD register on both wards (Nursing and Midwifery Council , 2014). The hospital coordinator must take the patient’s Medical Kardex from the ward requiring CD (Ward-X), to the ward holding stock of the required CD, (Ward-Y) The assigned Nurse /Midwife in charge of the ward holding CD, Ward-Y must authorized the removal of the required CD from their CD from their capboard. A registered nurse/midwife on Ward Y make an entry in the appropriate page of the Ward controlled drugs. Register belonging to Ward Y, indicating the stock has been transferred to Ward X. The entry should state, e.g. “2tablets tranfered to Ward X for patient A” (Nursing and Midwifery Council, 2014). This should be assigned by the nurse/midwife from Ward Y and countersigned by the Hospital Co-ordinator.The Hospital Co-ordinator will transfer the controlled drugs from Ward Y to Ward X. A registered nurse/midwife on the ward requiring the CD, Ward X, must make an entry in the appropriate page of the Ward Controlled Drugs Register belonging to Ward X, stating e.g. “2 tablets transferred from Ward Y for patient A” this should be assigned by the nurse, midwife from Ward X and countersigned by the Hospital coordinator. Ward/department Controlled Drug Registers should be chacked by pharmacy staff every three months following agree SOPs. The Appointed Registered Nurse / Midwife or Manager in Charge is responsible for the safekeeping of, and for controlling access to, all medicines stored in his or her area of control (Nursing and Midwifery Council, 2014). The keys should normally be held by the Assigned Nurse / Midwife in Charge of the ward / department. All CD issues will be recorded in the appropriate section of the pharmacy Controlled Drug Register, which should be maintained with a running balance total for each product. The following details must be recorded:
Name of the patient (if CD supplied against a discharge, pass or outpatient prescription). If the CD is supplied directly to the patient or their representative from pharmacy, the following additional information must also be recorded in the Pharmacy Controlled Drug Register:
Whether the person who collected the CD was the patient, their representative or a healthcare professional acting on behalf of the patient. If the person who collected the CD was a healthcare professional, that person’s name and address. If the person who collected the CD was the patient or their representative, whether evidence of identity was requested. (As a matter of good practice a note as to why this was not requested may be included but this is not mandatory.) Whether evidence of identity was provided by the person collecting the drug.
Date on which the supply was made. Ward or department. Name of the person requisitioning the CD who must be an authorised signatory for CDs for the area requesting it. Amount supplied. Form in which supplied. Serial number of relevant page in the Ward Controlled Drugs Order Book. Signature of the person supplying the CD
A computerised Controlled Drug register may be used provided safeguards are incorporated into the software to ensure all of the following:
The author of each entry is identifiable. Entries cannot be altered at a later date. A log of all data entered is kept and can be recalled for audit purposes. Access control systems are in place to minimise the risk of unauthorised or unnecessary access to the data and adequate backups are made.
The following points are important with respect to pharmacy Controlled Drug Registers:
All entries must be in chronological order. A separate part of the register must be used for each class of CD. The class of drugs must be specified at the top of each page. Entries must be made on the day of the transaction or on the next following day. No cancellation, obliteration or alteration may be made; correction must be by dated marginal note or footnote. Entries must be ink or otherwise indelible
The Ward Controlled Drugs Order Book must be signed and dated by the pharmacy personnel making the supply. All CDs will be supplied in a sealed tamper evident container, e.g. an Envopak bag with a numbered security tag
Accountable Officer
The Accountable Officer is responsible for the management of controlled drugs and related governance issues in their organization. Under The Controlled Drugs (Supervision of Management and Use) Regulations (Northern Ireland) 2009 HSC organisations, the armed forces and Relevant Independent Hospitals must appoint an Accountable Officer to be responsible for the management of controlled drugs and related governance issues in their organisation.
Patient Group Directors
There are currently only four circumstances in which CDs may be administered or supplied under a PGD: A registered nurse may, when acting in her capacity as such, supply or administer diamorphine under a PGD for treatment of cardiac pain to a person admitted as a patient to a coronary care unit or accident and emergency department of a hospital. A registered nurse / midwife, pharmacist or any of the other named healthcare professional listed may, when acting in their capacity as such, supply or administer any Schedule 5 CD in accordance with a valid PGD. A registered nurse / midwife, pharmacist or any of the other named healthcare professionals listed may, when acting in their capacity as such, supply or administer any Part 1 Schedule 4 CD in accordance with a valid PGD provided that it is not a drug in parenteral form for the treatment of addiction. A registered nurse / midwife, pharmacist or any other named healthcare professional listed may, when acting in their capacity as such, supply or administer midazolam in accordance with a valid PGD.
Nurse / Pharmacist independent and supplementary prescribers
The supplementary prescriber provides continuing care to the patient following assessment and diagnosis by the independent prescriber (who must be a doctor or dentist) and according to a CMP agreed with the independent prescriber.
The independent prescriber is responsible for:
The initial assessment of the patient, the formulation of the diagnosis and determining the scope of the CMP. Reaching an agreement with the supplementary prescriber about the limits of the responsibility for prescribing and review – which should be set out in the CMP. Providing advice and support to the supplementary prescriber as requested. Carrying out a review of the patient’s progress at appropriate intervals, depending on the nature and stability of the patient’s condition. Sharing the patient’s record with the supplementary prescriber.

The supplementary prescriber is responsible for:
Prescribing for the patient in accordance with the CMP. Altering the medicines prescribed within the limits set out in the CMP, if monitoring of the patient’s progress indicates that this is clinically appropriate. Monitoring and assessing the patient’s progress as appropriate to the patient’s condition and the medicines prescribed. Working at all times within his/her clinical competence and professional code of conduct, consulting the independent prescriber as necessary. Accepting professional accountability and clinical responsibility for prescribing practice. Passing responsibility for prescribing back to the independent prescriber if the agreed clinical reviews are not carried out within the specified interval, or if it is felt that the patient’s condition no longer falls within his / her competence. Recording prescribing and monitoring activity in the shared patient record as soon as possible.
EXPLAIN HOW DRUGS DEPENDENCE TREATMENT ARE SUPPLIED TO DRUG MISUSERS
FP10s for Schedule 1, 2 or 3 Controlled Drugs (CD) are valid for 28 days. Both FP10MDA and WP10MDA forms for instalments are limited to a maximum period of treatment of 14 days (Skaczkowski, et al. 2016). For instalment prescriptions, the prescriber must specify the instalment amount AND the interval between each instalment.Further information on dispensing Controlled Drugs can be found on the ‘Dispensing Controlled Drugs‘ .
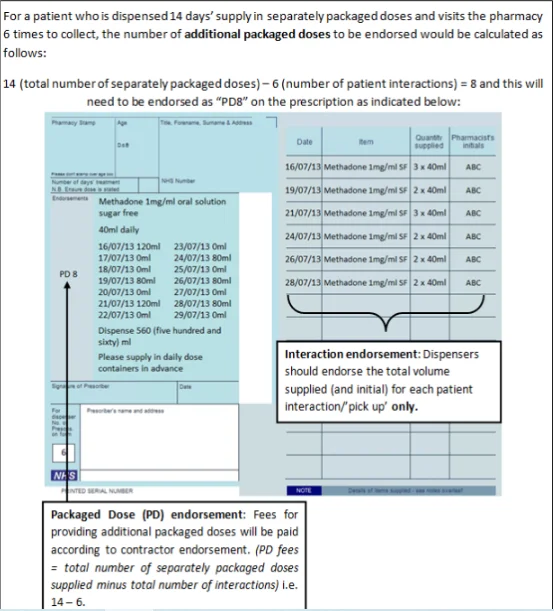
Endorsing
Pharmacists will endorse the prescription with the volumes dispensed at each “pick-up” episode (i.e. each time the patient collects their drug from the pharmacy).No endorsement is required to be paid for each “pick-up” or “item level fee”, these are paid automatically for all prescriptions for oral liquid methadone (including FP10 forms). An additional “Packaged Dose” fee of 55p can be claimed per additional bottle of oral liquid methadone supplied. The number of additional packaged doses claimed must be clearly endorsed on the prescription as payment of this fee will be based on the endorsement given only (NICE guidelines, 2016). The required endorsement is PD and the number of additional packaged doses supplied, e.g. PD 8 (see worked example that follows). The number of additional packaged doses supplied is equal to the total number of days’ supply given minus the number of patient interactions.
Work Example
References
Cavanagh S. (2018). Retention of Pharmacy Records. Specialists Pharmacy Services. East Anglia Medicines Information Service.
Dowell, J., et al. (2015). The UK medical education database (UKMED) what is it? Why and how might you use it? BMC Medical Education. 18(6): 2018.
Steele S, Adcock C, Steel A.(2016). Ethical, legal and professional issues arising from social media coverage by UK Helicopter Emergency Medical Services. Emerg Med J 2016;33:57-60.
Royal Pharmaceutical Society of Great Britain Medicines, Ethics and Practice(2016). A guide for pharmacists, Benhem & Company Ltd;
Nursing and Midwifery Council (2014). The NMC code of professional conduct: standards for conduct, performance and ethics (n.d).
Steve C. (2013). Counterfeit medicines: a cause for concern in the UK?
Official Journal of the European Union. 343(1)
OPiod dependence (2015). NICE CKS, April 2015. UK Access Only
Drug misuse and dependence (2017) - UK guidelines on clinical management, GOV, UK, 2017
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



