Influenza Vaccine in HIV Pregnant Women

Initially, the study yielded 617 records - 156 of which were duplicates. Afterward, the remaining studies were screened at the abstract and title stage, leading to the elimination of 67 records. On the other hand, 84 records were excluded at the full-text stage. For instance, 20 records were excluded because they were not written in the English language, including them could have made it difficult for the researchers to comprehensively understand and evaluate the data they presented. 15 records were also excluded because they focused on children born with HIV. The main aim of the study was to evaluate the effectiveness of the influenza vaccine on HIV-infected adults. Records that focused on children were therefore considered irrelevant. Twenty records were also excluded from the study because they focused on adults that were not HIV-positive. The main aim of the study was to evaluate the effectiveness of influenza vaccination among HIV-positive adults and therefore any study that focused on HIV-negative adults was considered irrelevant. Details of the full-text records excluded are illustrated in the figure below:
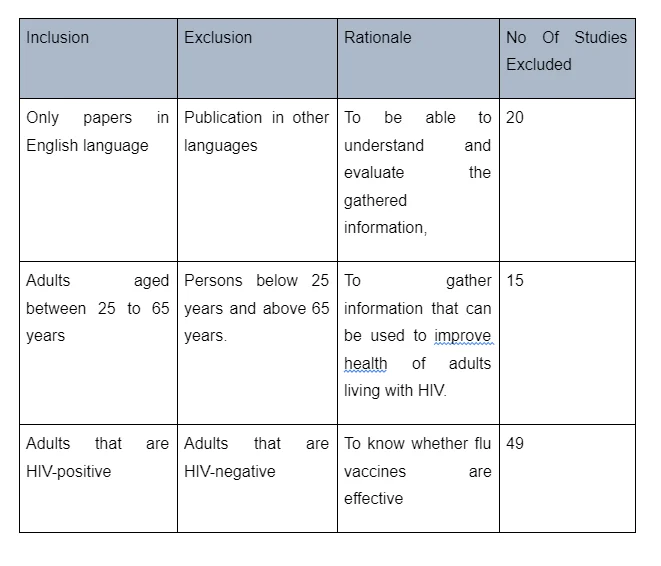
After exclusions and removal of duplicates, 5 studies met the eligibility criteria. All of them pertained to influenza vaccination among HIV-positive adults. The characteristics of eligible studies have been summarised in table 2. However, it is important to note that a majority of the selected studies were randomised control trials (n=3), while one was a cross-sectional controlled trial and the other one was a retrospective cohort study.
Characteristics of the study
Mark et al (2013) conducted a study to investigate the safety and immunogenicity of influenza vaccine in HIV-positive pregnant women. The study design was a randomised control trial that used a quantitative methodology for data analysis. The study only entailed women participants of age 18-39 years who were within the gestation period of 14-34 weeks. Only the participants who were on antiretroviral therapy beforehand or those who were concomitant with the first therapy were allowed to participate. On the other hand, participants who were allergic to eggs or any other element of the vaccine were not allowed to participate. The researchers administered two 30-μg doses of inactivated and unadjuvanted influenza vaccine. Haemagglutination inhibition was then measured at baseline, 21 days after the first dose, 10, and 21 days after the second dose – in infants and mothers at delivery and three and six months after delivery. Upon administering the 30-μg doses of inactivated and unadjuvanted influenza vaccine, the doses were found to be immunogenic in the HIV-infected women. The researchers did not observe any concerning safety signals. An efficient trans-placental antibody transfer was also noted, although seroprotection in infants rapidly waned. Hence, the study concluded that it was feasible to vaccinate HIV-infected pregnant women and their newborns from influenza. Tasker et al (2013) also conducted a study to explore the efficiency of influenza vaccination on HIV-infected persons. The study was a double-blind placebo randomized trial that included 102 participants infected with HIV. Data was statistically analysed using Statview 4.5 software, as well as the t-test. 55 participants were randomly assigned to receive the vaccine while 47 of them were randomly assigned to receive the placebo. The intervention was a characterised measurement of CD4+ cell, as well as plasma HIV-1 RNA levels at baseline, one month after trial and three months after the trial. The researchers concluded that the influenza vaccine was highly effective in HIV positive patients and that it does not have any association with any substantial change in CD4 cell count or viral load. Glinka et al (2016) took a different perspective with their study and were interested in identifying whether the timing of influenza vaccine affected its effectiveness in HIV-infected patients. To summarise, they explored whether there was a relationship between the incidence of influenza and timing of influenza vaccine in HIV-infected patients. The study took a retrospective cohort design and recruited HIV-infected patients under care from the San Diego Healthcare System. The researchers conducted a retrospective analysis of all charts of HIV-infected patients under care during the observational period. The researchers considered influenza to be present if there was a diagnosis of influenza by a physician or in cases where there was laboratory-confirmed influenza infection. The study findings indicated that patients who had received influenza vaccination earlier in the season were more susceptible to contract influenza as compared to patients who received influenza vaccine later in the flu season.

Ellis et al (2016) conducted a study to investigate the knowledge and use of influenza vaccine among HIV-positive patients. The study’s main target was to understand the uptake of influenza vaccine and the reasons why some patients decline the vaccine. The study’s objective was to explore whether the patients had any knowledge regarding the efficacy and effectiveness of the vaccine, as well as the clinical course of influenza among HIV-infected patients. The cross-sectional qualitative study involved participants from a HIV-positive routine care facility in London and was carried out between September and December 2014. The key data collected included the patient’s demographic data, the use of antiretroviral therapy, current and previous influenza vaccine use. Data was collected through a questionnaire method and the inclusion criteria were patients attending clinical appointments in pursuit of ambulatory care services. A total of 254 patients participated of which 195 were males, 39 females whilst 19 participants did not disclose their gender. The median age for study participants was between 55-64. A majority of the respondents in the study suggested that flu was more severe among people with HIV infections, while only 35% were confident about the efficacy and effectiveness of influenza vaccine in HIV-positive patients. In fact, 42% of the respondents believed that taking a flu vaccine did not necessarily mean that they will no longer contract influenza. Lastly, the study by Garg et al (2016) aimed to compare the immunogenicity of influenza vaccine in HIV-positive men who had sex with men. The study was based on the background that HIV-positive status increased the chances of contracting influenza, yet these patients where characterised by sub-optimal immune responses to standard dose influenza vaccine. The study design was a randomised double-blind control trial that was designed to compare the immunogenicity of standard dose intramuscular inactive influenza vaccine (IM) (15μg) versus off-level standard dose intradermal influenza vaccine (ID) in HIV-positive patients.
Dig deeper into Overview of the Medicines Act 1968 with our selection of articles.
The study recruited participants in a HIV clinic in Bangkok, Thailand. The inclusion criteria included Thai citizens that were HIV infected and were between the age of 18 and 60 years old. The study also recruited uninfected participants for the control study. At baseline, the participants were tested for HIV, CD4 count and HIV plasma RNA load. The same tests were conducted 1 month after vaccination. The participants were randomly selected and received the vaccination at the ratio of 1:1 of the IM or ID vaccine. In regard to the intervention, the researchers administered split-virion trivalent influenza IM and ID influenza. Both the study group and control group received 0.5-mL dose of IM vaccine and each 0.1-mL of IM vaccine was formulated to constitute 15 μg of HA in each virus strain of A/California/7/2009(H1N1)–like virus, B/Brisbane/60/2008–like virus and A/Perth/16/2009(H3N2)–like virus. Southern Hemisphere IM and ID vaccine was used to replace the Northern Hemisphere vaccine after the latter’s expiry. Ultimately, after administering both the ID and IM influenza vaccine to a total of 400 participants (i.e. 200 in the study group and 200 in the control group), the study found no significant difference in the immunogenicity of both standard dose IM versus ID influenza vaccine among HIV infected population of Thai origin.
Results of Methodological Quality Assessments
The main aim of evidence-based practice in medicine and nursing is to ensure the delivery of quality clinical services that are informed by and based on empirical evidence. To do so, nurses and other practitioners must be able to evaluate and assess the quality of research articles and reports to identify their strengths and weaknesses (Dawes et al, 2000). Dawes (2005) highlights that when evaluating a research paper, conducting a critical analysis helps in making valid judgments on whether the study results have been influenced by either the characteristics of the study design or the methodological process through which it was carried out. Against this backdrop, this section seeks to conduct a critique of the selected research papers included in this study. The critique will be conducted using various generic tools.
Study validity
Participants
Continue your journey with our comprehensive guide to A Looming Threat for Pregnant Women.
All 5 studies have clearly defined their inclusion/exclusion criteria for selecting participants. For instance, Glinka et al clearly state that their participants were drawn from the Department of Veterans Affairs. They included only the patients who had positive HIV status. On the other hand, they excluded participants who were HIV negative, as well as those whose influenza-like symptoms emanated from other diagnoses. This inclusion/exclusion criterion was appropriate for their research question because they were only interested in evaluating the effectiveness of influenza vaccine in HIV-positive patients and not HIV-negative patients. On the same note, Ellis et al state that they only included participants who were HIV-positive and were receiving routine care between the months of September and December 2014. Next, Mark et al only included HIV-positive women participants who were on antiretroviral therapy. They excluded participants who were allergic to eggs and those who were reactive to activated influenza vaccine. The same trend of clear statement of participant inclusion/exclusion criteria is evident in the study by Sybil et al, who included any HIV-positive patient who had not yet received their annual influenza vaccine; while Garg et al only enrolled patients of Thai citizenship, were HIV-positive, and between 18 and 60 years old. The effective identification and description of exclusion/inclusion criteria for all the 5 studies enhance the reliability of the enrolment procedures. Furthermore, it is important to note that all the five studies’ exclusion/inclusion criteria were appropriate for their respective objectives. For instance, all the studies were interested in HIV-positive patients and this formed part of their inclusion criteria. By only including HIV-positive patients, they were able to answer their respective research questions that were only pertinent to HIV-positive patients.
Interventions/comparisons
All the studies had well-defined interventions/exposures and comparisons, which were easily replicable. For instance, Glinka et al only considered influenza infection or influenza-like illness in existence only after gaining a laboratory confirmation or clinical confirmation, respectively. The laboratory confirmation was conducted through a laboratory screening test, which can be replicated. Likewise, Mark et al clearly illustrated how they administered their vaccine doses, and how they had complete specimen before delivery to provide ≥95% ability to detect any adverse events. In doing so, they used the Exact McNemar test to compare the rates of seroresponse, seroprotection and complete response within different time points. Sybil et al also provided a clear description of their intervention, i.e. influenza vaccine purchased by the hospital for clinical use was prepared in syringes and administered on both the placebo and vaccine recipients. Both of the two randomised controlled trials clearly illustrate how they randomised the assignment of the intervention to both the exposure and the comparison groups. For instance, Sybil et al explained that the preparation of vaccine syringes was conducted in a random computerized sequence that remained sealed until the end of the trial. The researchers also give a clear detail of the type of vaccines used, in a manner that enables any other researcher intending to conduct the same study to replicate. Likewise, Garg et al described that they randomly assigned both the ID and IM influenza vaccine to both the exposure group and the placebo group. Their randomization was first stratified depending on the participant’s CD4 count, but after the first 8 months, there was a substantial lagging of participant recruitment. This necessitated the dropping of stratified randomization to ensure that there was an adequate enrolment of participants. The clear description enables an easy replication of the study by any other researcher who would like to achieve the same objectives. However, in the two randomised controlled trials, the authors fail to identify whether the randomisation was concealed or not. The three other studies do not give details of randomisation of the treatment assignment to the study groups. Only four of the five studies clearly show the equality of their participants at baseline. Glinka et al used laboratory tests to ensure that all the participants had influenza infection at baseline, while Ellis et al only ensured equality at baseline using the inclusion/exclusion criteria because of the study’s qualitative nature. In relation to the two randomised controlled trials, Sybil et al and Garg et al successfully randomised their studies by ensuring that both the treatment and control groups were randomly assigned treatment by ensuring that they were similar at the start of the study. Nonetheless, all the two cohort studies and two randomised controlled trials have analysed the groups within which the participants were initially assigned. One cohort study (i.e. Ginka et al) employed a similar and valid measurement of variables in all the groups. For instance, Glinka and colleagues measured the timing of influenza vaccination by determining whether the vaccine was administered early or late in the season; while incidences of influenza or influenza-like illnesses were measured by whether they occurred either early or later in the season. These measurements were applied to all the groups. This criterion does not apply to Ellis et al.
The two randomised controlled trials clearly indicate that the health workers, researchers, and the participants were blinded to the exposures. For instance, the nurses in the study by Garg et al knew the vaccine assignments, all the other participants were blinded to the vaccine assignment until the end of the study. The study participants could view the vaccine as it was being physically administered but they were not aware of which group of vaccine they received. Likewise, the pharmacy staffs in the study by Sybil et al were blinded to the vaccine in the syringes because the syringes were generated through a computer sequence that was kept secret until the end of the study. However, the two cohort studies did not identify whether the staff or participants were blinded. Apart from the intervention, all the groups in all the five studies were treated equally. For instance, all the patients in Glinka et al were retrospectively analysed at baseline. In Ellis et al, all the participants were subjected to the same questionnaire. Sibyl et al ensured that all the participants gave their informed consent, while Garg et al ensured that all the participants had similar tobacco and drug use, socioeconomic status, HIV parameters and medical comorbidities. Secondly, all the participants in three of the studies were subjected to assessment at baseline and after the intervention. For instance, in the study by Mark et al, the patients were subjected to the assessment of adverse events. Thereafter, Mark et al conducted clinic visits to all the participants 72 hours within symptoms of influenza-like illness or fever, as well as within 24 hours within the onset of feet/hand tingling, the difficulty of walking, or lower extremity weakness. Likewise, Sybil conducted clinical assessments of all the participants at baseline by observing their CD4 count levels, influenza body titres, and plasma HIV-RNA levels to a baseline assessment. Lastly, Garg et al subjected all their participants to baseline assessment exposing their serum to 2-fold serial dilution tests. Mark et al, Sybil et al, and Garg et al developed measurement parameters and follow-up schedules in a similar manner for all their respective participants. Unfortunately, the two cohort studies, the two randomised control trials, and the cross-sectional study did not make any effort to measure the participants’ compliance with the intervention; neither did they indicate why they did not do so. It is therefore difficult to tell whether the participants sufficiently measured compliance with the intervention procedures.
Outcomes
Glink et al did not clearly define their outcome measures, considering that it was a retrospective cohort study. However, it is important to note that the study considers patients with influenza or influenza-related illness as those who were clinically tested had a positive laboratory test. This was similar to the case of Ellis et al, whose qualitative nature prohibited the use of outcome measures. However, Ellis et al made reference to the participants’ reporting to measure several variables such as immunization rates. In their cohort study, Mark et al measured vaccine outcomes by examining infant Haemagglutination Inhibition (HAI) titres at birth and at 3 months. The authors measured infant and maternal titres 6 months in mother-infant pairs after receiving both complete sets of sample and doses of vaccine before delivery. The use of HAI titres as an outcome measurement is easily replicable as it can be conducted by any other researcher intending to conduct the same type of study. On the other hand, Sybil et al measured their outcomes based on the participant’s CD4 cell counts, HIV-1 RNA levels and influenza antibody titres post-vaccination to evaluate the vaccine effectiveness. All the studies except Ellis et al (which cannot be evaluated based on this criterion because it is a cross-sectional study) conducted an adequate, complete and sufficient follow-up on the subjects under investigation. For instance, Glinka et al followed up the patients considered to have received late vaccination, to identify whether they had been infected with influenza or developed any influenza-like illness. The follow-up was sufficient as it covered all the targeted participants. Being a retrospective cohort study, there were no incidents of participant dropout reported. The cross-sectional study by Ellis et al did not report any follow-up, nor were there any reports of participant drop out. Garg et al also conducted a sufficient, adequate and complete follow-up of both the placebo and the treatment group. The follow-up began immediately after vaccine administration, whereby there was a standby physician who conducted a safety oversight and interim analysis of all the participants to ensure that all the cessation criteria were met. Garg et al also conducted post-vaccination monitoring (i.e. 1 month after vaccination) to identify any antibody responses among all the treatment groups. The follow-up was sufficient enough to identify no significant difference among the vaccine groups in regard to seroprotection and seroconversion among participants. Sybil et al also conducted a complete and sufficient follow up of all patients one and three months after vaccination. The follow-ups entailed the measurements of the participant’s CD4 cell counts and HIV-1 RNA levels, as well as for serologic influenza examination. The follow-up conducted by Sybil et al also involved an interviewing of all the participants to identify any respiratory illnesses they experienced during the study period. An equally sufficient and complete follow up assessment was conducted by Mark et al whose main target in the follow up was the infants, an exercise through which one infant was detected to be HIV positive. However, all the five studies did not indicate whether they blinded all the outcome assessments. Based on these analyses, it can be concluded that the five studies had a good quality of study design and all effort had been made to minimise bias. The following table summarises the results of the study design quality evaluation:
Study Validity Checklist

Quality of study results
The two cohorts (Glinka et al and Mark et al) studies and the two randomised controlled trials (Garg et al and Sybil et al) reported their measures of occurrences and intervention effects. Four of the studies reported the number of participants in both the comparison and the exposure group, the proportions in outcomes in each group and the relevant measures of the vaccine effects. This enabled the ascertainment of the accuracy of the vaccine effects in terms of risk differences, mean differences, or relative risks. All the studies reported their measurement of precision by using either p-value or confidence intervals. For instance, Glinka et al, Garg et al, Sybil et al, and Mark et al used p-value to measure the precision of effects while Ellis used a confidence interval to achieve the same. Both the cohort studies and the randomised controlled trial studies calculated and reported the useful effect estimate. It was evident in all the four studies that these estimates were used to give some meaning to practice. For example, Glinka et al conclude that HIV-positive patients who received the vaccine earlier in the season were significantly more likely (i.e. p0.01) to contract influenza or influenza-like illness than those who received the vaccine later in the season. Based on this effect estimate, Glinka et al concluded that in practice, HIV-positive patients are more likely to contract influenza if they received the vaccine earlier in the season. All four studies measured the effects estimates for both benefits and harms of the influenza vaccine. All the studies used sufficient estimates of precision of effects. Ideally, Ellis et al’s confidence intervals were narrow and did not include the ‘no effect point’. The rest of the studies (all of which used p-value to estimate vaccine effects) had p-values 0.05, therefore their estimates were likely to be good and sufficient due to sample size being big enough. Nonetheless, the two randomised controlled trials were mono-centred (i.e. they were conducted within a single site); hence the researchers did need to increase the confidence in the results. All in all, the quality of all the five results can be judged as precise and useful, which may be expected of RCTs.
Study Applicability
All the five studies properly described their participants; therefore, it was easier to assess the generalisability of the study findings to the HIV-positive target group. It was easier to establish that the study participants across all the studies were a typical or atypical representative of the HIV-positive population. It was also easier to determine the similarity/relevance of the participants to the HIV-positive group as the specific target group. Upon ascertaining this similarity or relevance, it was easier to determine the applicability of the influenza vaccine to the HIV-positive population. All the studies also clearly described the applicability of influenza vaccine intervention within the test and in the reference. There was a theoretical possibility of replicating all the five studies. In regard to exposures and comparisons, all the cohort studies and the randomised controlled trials did not administer the intervention to the comparison group (i.e. they only received placebo). This made it impossible to determine the comparison group management.
A Narrative Synthesis of Results
The majority of the studies aimed to identify the general effectiveness of influenza vaccination in HIV-positive patients. While all the studies did not make a clear description of the theoretical basis for influenza vaccination in HIV-positive patients, the implicit theory underlying most influenza vaccine intervention for HIV-positive patients is that HIV infection exposes the patient to more vulnerability and risk to influenza infection. The use of influenza vaccine as an intervention to influenza infection or influenza-like illness is an indication that the authors believe in the importance of vaccination in HIV-positive patients against most infectious diseases. Two of the studies were randomised control trials (RCT) whereby in one RCT (Sybil et al) the intervention group received the vaccine while the control group received placebo, whereas in the other RCT (Garg et al), one study group received standard-dose intramuscular influenza vaccine while the other study group received intradermal trivalent inactivated influenza vaccine. However, one cohort study (Mark et al) conducted a pre- and post-intervention analysis.

Intervention Content
Mark et al, at entry, subjected their participants to a 30 μg/dose inactivated pH1N1 monovalent flu vaccine through two standard injections of 15 μg/0.5 mL, each administered per upper extremity or with a 2 inches difference in the same upper extremity. The participants who did not encounter any adverse event (i.e. ≥3) or those who did not develop any response that satisfied the exclusion criteria were given a 2nd dose of 30-μg after seven days. Ideally, Mark et al chose a double dose regiment for purposes of easier establishment of immunogenicity. The researchers then assessed the participant’s haemagglutination inhibitors at baseline, 10 days and 21 days after vaccination. The intervention by Sybil et al involved an administration of the saline vaccine (control group) and influenza vaccine (for intervention group) using randomly assigned syringes. The syringes were randomly assigned by a computer system and the sequence of randomization was not revealed until the end of the study. Each syringe contained a whole virion with A/Texas/36/91 [H1N1], A/Johannesburg/33/94 [H3N2], and B/Harbin/ 07/94 each of 15 μg. Participants returned at 1 and three-months post-vaccination for follow-up measurements of CD4 cell count, for serologic examination and for HIV-1 RNA level measurement. On the other hand, Garg et al administered both ID and IM influenza vaccine. The vaccines were both split-virion, inactivated, and trivalent. The Northern Hemisphere vaccines contained influenza streams of both B/Brisbane/60/2008–like virus and 16/2009(H3N2)–like virus. Each 0.5-mL dose of IM and ID influenza vaccine was formulated to contain 15 μg of hemagglutinin for each listed strain. The researchers replaced the expired Northern Hemisphere IM and ID vaccines with Southern Hemisphere vaccines, which were used for the rest of the study. Importantly though, both vaccines were of the same composition. On a penultimate basis, Glinka et al retrospectively analysed HIV-infected patients in respect to their influenza infections as well as any influenza-like illness presented in them. The researchers categorised the influenza vaccination in line with what they considered late vaccination and early vaccination according to influenza outbreak seasons and trends in California. The researchers then evaluated the CD4 count and viral load of both the late and early vaccinated groups.
Effectiveness Measurements
Effectiveness measurements were disclosed in all the studies except the cross-sectional study. For instance, Glinka et al intended to measure the effects of timing on the effectiveness of influenza vaccine in HIV-positive patients. In doing so, the study used both viral load and CD4 counts to determine whether patients who had received late or early influenza vaccine had the same level of effectiveness of influenza vaccine. Secondly, Ellis et al did not entail any measurement of effectiveness, while Mark et al reported their effective measurements. Beforehand, it is important to note that Mark et al aimed to investigate the safety and immunogenicity of influenza vaccine (2009 pH1N1) in HIV-positive pregnant women. Against this backdrop, they measured the effectiveness of the vaccine through HAI titres i.e. by measuring the occurrence levels of seroresponse and seroprotection after each dose, as well as the presence of seroprotective titres. Next, Sybil et al measured the effectiveness of the influenza vaccine by testing the HIV1-RNA levels and CD4 counts in both the treatment group and the placebo group. In detail, the CD4 cells percentage variation, as well as the patients’ plasma samples (for measuring HIV-1 RNA) were subjected to laboratory tests 1 month and 3 months after vaccination, and the results were statistically analysed to compare the HIV-1 RNA levels and CD4 count levels between the placebo and the intervention groups. Garg et al aimed to make a comparison of the immunogenicity of IM versus ID influenza vaccine in HIV infected men having sex with men in Thailand. They compared the immunogenicity of the two influenza vaccines by measuring the seroconversion and seroprotection titres of each vaccine one month prior to, and one month after administering each vaccine. The following table summarizes the study results as well as other characteristics of each study:






- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



