Targeting AML: Evaluating the Antileukemic Efficacy of QUB-1518 Peptide
ABSTRACT
Acute myeloid leukemia (AML) is a blood cancer that is caused by a reaction of the clonal expansion of blasts of the myeloid lineage that have differentiated abnormally. This disease is classified under hematological malignancies comprising the most common acute leukemia in adults. Currently, Acute Myeloid Leukemia poses a major threat to the public as there is an emergence of approximately 7000 cases annually at the United Kingdom alone with over 4000 deaths. Leukocytosis, bone marrow failure, fatigue and anorexia are some of the signs and symptoms of AML. Diagnosis of the disease is done by examining a bone marrow biopsy, doing a full blood count or tracing the origin of the AML cells. Even though there are several treatment options ranging from induction therapy, allergenic hematopoietic stem cell transplant (HSCT) and chemotherapy based on clinical findings and age among other factors, the degree of prognosis is still poor. This has necessitated for the research and development of alternative methods of treatment of AML. Anti-microbial peptides have recently drawn the attention of scientist as possible chemotherapeutic agents. This study aims to examine the anti-leukemic effects of the QUB-1518 against the AML MOLM-13 cells. An experiment to test the antileukemic effects of the peptides against the AML MOLM-13 cells. Cell proliferation of the MOLM-13 was assessed using the MTS assay and the cells exposed to the QUB-1518 peptide for a period of 24 hours and 48 hours respectively. Results obtained depicted that there was cell death due to the decline in MOL-13 cell viability over time. The QUB-1518 cytotoxic mechanisms of actions are not fully understood but cytotoxic activity possibly occurred through disruption of the cell membranes, mitochondrial and lysosomal disfunction and/or disruption of the cellular metabolite content. QUB-1518, therefore, qualifies to be a potential chemotherapeutic candidate for the treatment of acute myeloid leukemia. For students pursuing research in biomedical science dissertation help, make sure to explore the therapeutic potential of peptides like QUB-1518 as it offers valuable insights into novel treatment avenues.
Introduction
Incidence and survival
Acute myeloid leukemia (AML) is a group of hematological malignancies comprising the most common acute leukemia in adults (Short, Rytting, and Cortes, 2018). It is a disease that develops as a result of a reaction of the clonal expansion of abnormally differentiated blast of the myeloid lineage. Signs and symptoms of the disease include abnormal infection and differentiation of clonal concentration of myeloid stem cells. These leukemic cells begin to develop in the bone marrow cells and dominate over the healthy cells in the bone marrow, as a result to the inadequacy of red blood cells, neutrophils, platelets and lymphocytes as their formation is not taking place due to this dominating red blood cells (Kuykendall, Duployez, and Boissel, 2016).

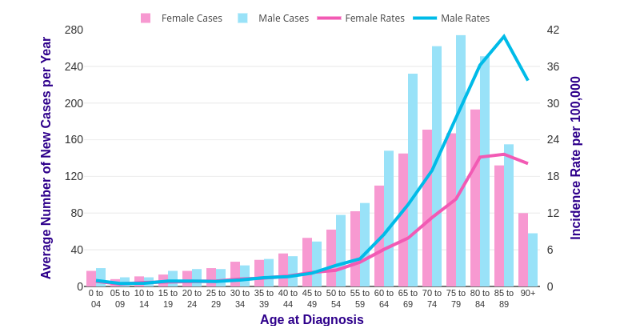
Even though there have been major recent therapeutic advancements over the past couple of years, AML is still a major global public health problem. There are an estimated 7000 new cases every year in the UK and kill over 4000 people each year. The number of deaths depicted worldwide was 309, 000 in 2018 (CDC, 2018). All incident cases of AML were identified in the cancer Research UK registry data. The study assessed the incident and mortality trends of AML in the UK in 2015-2017. Data were obtained from the Cancer Research UK (CRU). Standardized rates of incidence per 100,000 people were evaluated using the direct standardization method. Results indicate that incidence rates increased with an increase in age; the lowest rate was recorded among the youngest age groups, while the highest rate was recorded among the oldest age groups. Age specific incidence rates rise steadily from around age 50- 54, and it sharply increases between age group of 60- 64 and declined in the oldest age group. Males and females aged group 85 to 89 have the highest incidence rate. AML was significantly higher in men as compared to women in all examined age groups.
Pathogenesis
Because it is a bone marrow disease, AML has hematopoietic precursors that are arrested in the initial development stages. Both classes of mutation (Class I and II) must occur in conjunction with each other. Class I mutations activate proliferative pathways, while class II mutations result in the impairment of hematopoietic differentiation that causes the development of leukemia. Generally, Class I mutations like FLT3 (internal tandem duplications, ITD, and tyrosine kinase domain mutations, TKD), TP53, K/NRAS, and c-KIT are present in ~28, 8, 12, and 4% of cases, in that order. Research on hematological malignancies highlighted the function of activator of transcription 3 (STAT3) and signal transducers that stimulate cell proliferation and survival. Improved tyrosine phosphorylation of STAT3 due to enhanced cytokines secretion, like IL-6 or mutations in receptor tyrosine kinases (for instance, FLT3 duplications or less frequently JAK2) is seen in approximately 50% of AML cases. This is an indication of a worse prognosis. Examples of class II mutations are CEBPA and NPM1. The two classes are found in approximately 6% and 27%, respectively, and guarantee a better prognosis. Replacing genes involved in epigenetic regulation is considered as a 3rd class mutation as it affects both proliferation and cellular differentiation. Examples include TET2, IDH-2, mutations in the DNA-methylation related genes DNMT3A, IDH-1, and IDH-2, The above examples are available in more than 40% of AML cases (De Kouchkovsky and Abdul-Hay, 2016).
The existing pathophysiology of AML involves bone marrow cell maturational arrest during the early development stages. This mechanism involves the use of chromosomal translocation and epigenetic or other genetic abnormalities to activate and inactivate genes. The description of the molecular pathogenesis of AML has significantly improved over the last years. There are two major processes that are important for leukemic transformation include alteration in myeloid transcription factors controlling hematopoietic differentiation and activating mutations of signal transduction intermediates. The two processes are complementary since the molecular factors that change the transcriptional control in hematopoietic progenitor cells change the composition of signal transduction molecules that help in the growth of factor receptors. Similarly, activating mutations in signal transduction molecules stimulate changes inactivity and the expression of various transcription factors that are important for the differentiation of normal myeloid (Steffen et al., 2005)

Clinical Features
Most of the clinical diagnosis of AML shows the accumulation of malignant, poorly differentiated myeloid cells within the bone marrow, peripheral blood, and infrequently in other organs. Most patients develop both leukocytosis and bone marrow failure signs such as neutropenia, anemia and thrombocytopenia. Weight loss, Fatigue and anorexia, are also common symptoms. Lymphadenopathy and organomegaly are not very common. If left untreated, it may lead to death within months of diagnosis due to bleeding and infection (De Kouchkovsky and Abdul-Hay, 2016).
Laboratory investigations and diagnosis
The diagnosis of AML is established by demonstrating involvement of 20% or more blasts in the bone marrow or peripheral blood. It can also be diagnosed by tracing the myeloid origin of the cells by testing for myeloperoxidase cell activity, documenting the presence of Auer rods or immunophenotyping. These findings consist of azurophilic; usually, a needle-like cytoplasmic body inclusion commonly found in APL, AML, and most of AML with t (8;21). MLA can also be diagnosed with recurrent genetic abnormalities t (8;21), inv (16) or an extramedullary tissue infiltrates, t (15;17) in which the presence of the genetic abnormality is diagnosed, regardless of the blast percentage (De Kouchkovsky and Abdul-Hay, 2016).
Full blood count and blood film tests: The test is performed to check whether an individual has too many white blood cells compared to both the red blood cells and the platelets (Kuykendall, Duployez, & Boissel, 2016). If that is the case, the individual is diagnosed with AML. Moreover, if the blast cells are found in the bone marrow rather than in the blood, it can also confirm AML.
Bone marrow biopsy and/or aspiration
The process involves extracting samples of bone marrow from a patient's born for testing. A small needle is inserted inside the born to collect born marrow tissues. The sample is then viewed in the laboratory using the microscope to investigate AML.

Treatment
Approach to treatment is based on clinical findings, flow cytometry, cytogenetic and molecular genetic analysis where the condition can be subdividing by risk groups.
Patients diagnosed with AML first undergo induction therapy to achieve complete remission (CR). Despite this, minimal residual diseases often remain in CR. Therefore, if treatment is stopped, Lapse occurs inevitably. Moreover, a more effective therapy such as consolidation therapy should be considered along with induction therapy to get rid of any sort of residual disease and obtain a lasting remission. Induction therapy mainstay consists of '7+3' regimen, which is a combination of 7 days of infusion cytarabine plus three days of anthracycline. Generally, this is given to patients who show a sign of favorable prognosis and a low risk of TRM. Examples of such patients include younger patients, such as those under 60, with good recovery records, normal albumin, creatinine, and platelet count.
An effective remedy for elderly people with AML is yet to be established. Elderly people, above the age of 65 years, present a higher cytogenetic-risk profile. They have a low response to chemotherapy and are highly vulnerable to treatment-related toxicities. Although they exhibit poor prognosis, induction therapy has been proved to be effective in improving their rates of survival when compared with palliative chemotherapy and supportive care. Consolidated therapy should be offered to patients in remission to get rid of residual disease and prevent relapse.
Available options for consolidation include allergenic hematopoietic stem cell transplant (also-HSCT) and chemotherapy. When evaluating these options, it is important to consider the risk of TRM against the risk of treatment relapse or failure. According to Intention-to-treat analyses (assigning patients to chemotherapy or allo-HSCT according to the related-donor availability) has been proved to be not effective in allo-HSCT as compared to chemotherapy in patients with cytogenetically favorable AML in first CR. Therefore, chemotherapy is the best treatment option for patients with signs of a favorable prognosis. Generally, its regime consists of intermediate-dose cytarabine (two to four cycles, each consisting of six doses at 1.5–3 g/m2). This has also been proved to be effective in high doses.
Prognosis
Accurate assessment of prognosis is central to the management of AML. The prognosis and survival of AML patients depend on various factors. Only physicians with knowledge of prognostic factors can decide between increased treatment intensity or standard, allogeneic hematopoietic stem cell transplant or consolidation therapy. AML treatment appears to be complicated, with an increase in age and poor performance status, which may result in failure to achieve remission and decreased overall survival (Saultz and Garzon, 2016).
Patients are classified into favorable, intermediate or adverse prognostic risk groups based on their cytogenetic profile alone. All the t(8;21), t(15;17), and inv(16) chromosomal changes observed in AML confer a favorable prognosis with a 3-year overall survival (OS) rate of 33% and 66% at the age of 60 and above (Saultz and Garzon, 2016).Cytogenetic abnormalities such as a complex karyotype (several chromosomal abnormalities such as deletions of part or all of chromosomes 5 or 7, t(6; 9), or inv(3) and the long arm of chromosome 11 have a poor prognosis resulting in treatment failure and death (De Kouchkovsky and Abdul-Hay, 2016).
Although AML can occur in any age group, it affects most commonly adults with a median age of onset at ≈ 65 years. The overall survival rate of 5 years remains poor at 5% for patients >60 years. However, it has been marginally improved to 50% in younger patients. It has been established that older patients who did not receive intensive chemotherapy without unacceptable side effects remain dismal, resulting in more early deaths compared with the younger age group (Estey, 2018). Moreover, poor responses to chemotherapy have been found to have a poor outcome for AML patients. One of the major problems in cancer chemotherapy is that the existing chemotherapeutic drugs are toxic to cancerous cells as well as to normal cells. Such unwanted side effects lead to treatment delay, dose reduction, or discontinuance of therapy; therefore, it may sometimes cause the death of patients. Therefore, there is an urgent need to develop novel safe and effective drugs for AML treatments (Kuykendall, Duployez, & Boissel, 2016).
Cell Counting
In many scientific research and assays, it is crucial to acquire an accurate number of cells of the sample that is being dealt with, this is referred to as cell counting (Marie et al., 2005). Cell counting is essential in determining the health condition of a patient, examining the growth of microorganism in a culture, in quantifying the cell viability whereby the number of viable cells and dead cells in a culture is calculated. This helps in evaluating the life expectancy of a malignant cell (Xie et al., 2018). There are several methods and tools used in cell counting, a hemocytometer is one of the effective and efficient devices utilized in cell counting (LeGresley & McDermott, 2010)
Conclusion
In the search for novel anticancer therapeutics, antimicrobial peptides (AMPs) have been found to have anticancer effects, and recently, it has received much more attention as alternative chemotherapeutic agents. Therefore, AML has a relatively low long-term survival rate, and conventional chemotherapy has side effects, so it is necessary to develop novel drugs with less toxicity and higher efficiency.
Hypothesis and Aims
Cationic antimicrobial peptides (AMPS) have shown antibacterial, wound healing, antioxidant and antifungal properties. Moreover, in the search for novel anticancer therapeutics, AMPs have also been shown to have anti-cancer effects and recently, it has received much more attention as alternative chemotherapeutic agents. The AMP QUB- 1518 Peptide has anti leukemic effects against the AML (MOLM-13) cells.
The aims of the study were: To examine the anti-leukemic effects of the QUB-1518 against the AML MOLM-13 cells; To evaluate the cytotoxic mechanisms of actions of the QUB-1518 against the AML MOLM-13 cells; To understand if the QUB-1518 has potency against normal cells.
Methods and Materials
Materials
The following materials were used for the experiment

Methods
Cell culture
The acute myeloid leukemia cell line used in this study MOLM-13 was purchased from the DSMZ-German Collection of Microorganisms and Cell Cultures, and these cells were maintained in RPMI 1640 media at 37°C in the incubator, supplemented with 10% fetal bovine serum – FBS (From GIBCO, Brazil; Ref; 10270-106) , 100 U/mL penicillin, and streptomycin. The cell should be passage when RPMI 1640 media was prepared using 10% of FBS and Penicillin- Streptomycin (from Hyclone laboratories 925 West, 1800 South Logan, Utah), adding into the RPMI 1640 (from SMZ-German Collection of Microorganism and Cell Cultures).
2, 5-diphenyl Tetrazolium bromide (MTS) assay
MTS assay is a colorimetric sensitive qualification to assess the proliferation of cells in a culture media. The MTS assay kit was purchased from Abcam. AML cell line (MOLM-13) was plated into 96 well plate, following this an MTS assay was added on all the wells 20 µl.
Reagent Assay Preparation
2,5-diphenyl tetrazolium bromide (MTS) assay was dissolved in water at a concentration of 10 mg/ML; then, the sterilizing solution was filtered after adding the 2,5-diphenyl tetrazolium bromide Solution. The 2,5-diphenyl tetrazolium bromide solvent was prepared with 4mM HCl, 0.1% NP40 in isopropanol.
MTS Assay method
On day 1, cells were grown in fresh media. Day 2 comprised of seeding the cells, at a concentration of 2.5× 104 cells/well in 180µl culture medium into 96-well microplates in the presence of different concentrations of QUB-1518 (1.75, 3.75, 7.5, 15, 30, 60, μM), or without Peptide (control) and a positive control using 5μM doxorubicin. The final volume of culture medium was (200 µl) in each well. Doxorubicin was bought from Sigma – Aldrich. To ensure viability, each concentration utilized two wells, and controls were also established in 4 wells.
The micro-plates were incubated for 24 hours and 48 hours respectively in the incubator, after which 20µl of the MTT Labelling reagent was added to each well, the micro-plates were incubated for 4 hours in the incubator, at 37 °C. Plates were read after 4 hours of incubation. Complete solubilization was assessed by checking for purple formazan crystals and measuring the spectrophotometric absorbance using the FLUOstar Omega microplate reader at a test wavelength of 490nm. The results were recorded with the viability of the negative control cells taken as 100% cell survival. Statistical techniques such as mean, standard deviations and the Quest Graph™ IC50 Calculator were
RESULTS
To test the anti-leukemic effects of AMP QUB 1518, the AML MOLM-13 cells pol exposed to the peptides for a period of 24 hours and 48 hours respectively. The concentration range of the peptide was 1.75 µM to 60 µM
The Quest Graph™ IC50 Calculator was used to calculate the IC50 values, using the equation below:

The Hill coefficient value is 1.908 at 24 hours exposure while it is 2.988 at 48 hours exposure. This is automatically generated by the Quest Graph IC50 calculator.


The mean cell viability values were obtained at different intervals during the 24-hours period and the standard deviation calculated using Microsoft excel spreadsheet software in order to summarize this continuous data. Doxorubicin was used as a positive control in the experiment.
A curve was plotted to show the effect of the QUB1518 against mean cell viability within 24 hours as shown in Figure 4 below. The concentration of QUB 1518 ranges from 1.75 μm to 60 μm. This curve shows the mean cell viability has decreased gradually from 99.66 percent to 48.71 percent.

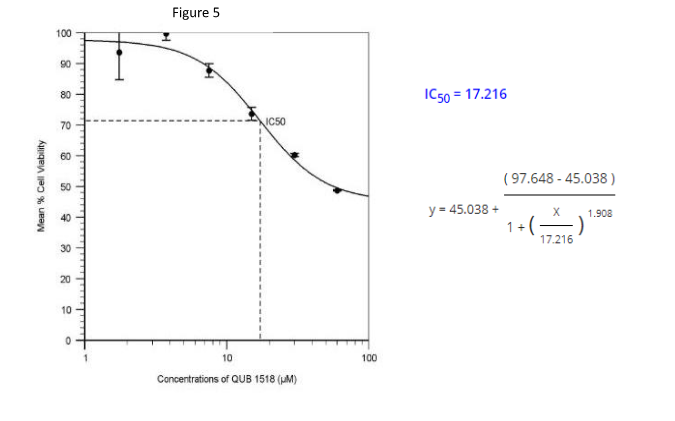
A dose-response curve of QUB 1518 effect on AML MOLM-13 cells is shown in Figure 5. The Quest Graph IC50 calculator was used to obtain the IC50 value below. The IC50 was calculated to be 17.216 µM under the conditions tested at a 24-hours exposure period.

The mean cell viability values were obtained at different intervals during the 48-hours period and the standard deviation calculated using Microsoft excel spreadsheet software in order to summarize this continuous data. Doxorubicin was used as a positive control in the experiment.
A curve was plotted to show the effect of the QUB1518 against mean cell viability within 48 hours as shown in Figure 6 below. The concentration of QUB 1518 ranges from 1.75 μm to 60 μm. This curve shows the mean cell viability has decreased from 91.61 percent to 20.50 percent.

A dose-response curve of QUB 1518 effect on AML MOLM-13 cells is shown in Figure 7. The Quest Graph IC50 calculator was used to obtain the IC50 value below. The IC50 was calculated to be 13.816 µM under the conditions tested at a 48- hours exposure period.

A double-horizontal bar graph as shown in figure 8 below was generated to compare the mean cell viability of the MOLM-13 against the concentration QUB 1518 at exposure periods of 24 hours and 48 hours respectively.

The viability of MOLM-13 cells declined gradually as the concentration of QUB-1518 increased, as shown in figure 4 and 6. The decline in cell viability was less in the 24-hour period as compared to a higher decline in the 48-hour period (Figure 8).
DISCUSSION
Biomaterial components such as peptides that harbor antimicrobial properties are found in many species; mammals, insects, amphibians and fish. They play a critical role in the host inborn immunity to microbial pathogens (O’Connor et al., 2013). In mammals, most of these peptides specifically target cells that are cancerous in nature and leave out the normal cells while other peptides are highly cytotoxic against both the normal and the cancerous cells. Peptides like the QUB 1518 contain a lipopolysaccharide (LPS) neutralizing capability and are can also cause an adaptive immune response to occur (Hoskin & Ramamoorthy, 2008). Apart from this, the peptides can also participate in the process of wound healing, apoptosis and immune modulation. This makes them the suitable anticancer agents (Zhang et al., 2019).
The MTS Assay
The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-4.5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide (MTS) assay is a colorimetric screening assay that is basically utilized to assess the proliferation of cells by measuring the activity of dehydrogenase enzyme found in cells that are metabolically active. The assay basically quantifies the cells that are viable in a cell proliferation assay. (McGowan et al., 2011). In cells that are metabolically active, enzymes reduce compounds of MTS Tetrazolium into a colored Formazan which is soluble in cell culture media (Arab et al., 2016). Since MTS can be converted into a colored and soluble formazan product, the color intensity yielded will be significant which can be easily quantified (Riss et al., 2013).
By utilizing MTS assay in this study, it was demonstrated that prolonged periods of incubation led to high intensity inhibition of cell proliferation (Arab et al., 2016). The period of incubation was limited, because the detection reagents are cytotoxic in nature and use up energy from the cell to produce a signal. The viable MOLM-13 cells in the experiment converted the MTS tetrazolium compound to a formazan product that is colored and soluble in nature (Yan et al., 2018) which could easily be quantified (O’Connor et al., 2013).
The antileukemic effects and possible mechanisms of action of QUB-1518
Cytotoxicity is the ability of a compound to cause variations in the behavior of a cell and disrupt fundamental processes that induces cell death or leads to a huge decrease in survival of the cell (Damiani et al., 2019). From the results above, the QUB-1518 peptide demonstrated cytotoxic activity against the MOLM-13 leukemia cells with concentrations ranging from 1.75 to 60 μM. Cell death of MOLM-13 was detected since the cell viability gradually declined as the concentration of peptide increased along with prolonged time. The cytotoxicity of the peptide was evidently dependent on the concentration of the peptide itself and the time factor. Putting up a concentration of peptides ranging from 1.75 μm to 60 μm over a period of 48 hours led to more cell death as compared to putting up the same concentration of peptide in a shortened period of 24 hours only. The longer the time, the more the cells were intoxicated. The IC50 value of the peptide QUB-15818 at 24-hour exposure was higher in comparison to the IC50 value at 48-hour exposure.
Various endpoints were used in the determination of cytotoxicity. They included: mitochondrial functions, permeability of the cell membrane, cell death, cellular metabolite content and lysosomal functions (Trivedi et al., 2016).
The Inhibitory Concentration value (IC50) is the concentration of drug needed to inhibit a viable cell number by 50% (Buri et al., 2017). A high IC50 value indicates that the viable cells are less sensitive to the drug whereas a lower IC50 value indicates that the viable cells are highly sensitive to the drug (Damian et al., 2019). The IC50 values for the QUB-1518 peptide were obtained after 24 hours and 48 hours as shown in figure 5 and figure 7 respectively.
To calculate the IC50 values, a quest graph IC50 calculator was used. The hill coefficient (nH) values were positives of 1.908 at 24 hours and 2.988 at 48 hours respectively. The hill coefficient quantifies the effect of cooperative binding between ligands and macromolecules (Abeliovich, 2005). The greater than one (>1) value indicates that there was positive cooperativity in binding. The nH values, in this case, were positive since this is a biological inhibition process.
At 24 hours, the IC50 for QUB-1518 was 17.216 and 13.816 at 48-hour exposure (Figures 5 and 7, respectively). These values obtained indicate that the sensitivity for MOLM-13 cells gradually increased as the exposure time was prolonged, therefore at 48 hours exposure, the cells were highly sensitive to the QUB-1518 as opposed to the 24-hour exposure, which had a higher IC50 value. The longer the time, the more the cells were intoxicated. The IC50 value of the peptide QUB-15818 at 24-hour exposure was higher in comparison to the IC50 value at 48-hour exposure.
The IC50 values are also valuable in measuring the resistance of a malignant cell towards a drug and determining the potency of a drug/ anti-microbial peptide against malignant cells. (Damian et al., 2019).
Though the mechanism of action of the peptides against the leukemic cells that results to cell death is not fully understood, Paredes-Gamero et al., (2013) demonstrated several experiments that could possibly explain what happens. Some peptides, such as the polybia-MPI, were found to cause cell death by necrosis without having any impact on normal fibroblasts. In addition to this, tachyplesin, a peptide that contains a disulfide bridge which forms a stable amphipathic Beta sheet structure and demonstrates antitumoral activity, also inflicts apoptosis in the cells by emanating potassium ions (Etchin et al., 2013).
A study performed by Etchin et al. (2013) indicated that leukemic cell death was characterized by the integrity of the cell membrane being destroyed and leakage of cytoplasmic content which represent necrosis. The plasma membrane of a tumor cell, having a critical role in the anticancer action of a peptide, is usually the first one to interact with the peptide (Snauwaert et al., 2013). This makes it permeable to the peptide and allows those that have immense hydrophobicity to infiltrate further into the core of the cell, which is hydrophobic (Zhang et al., 2019). The possible mechanism of action of the QUB-1518 is not fully known, but since it is evident from the experiment that cell death occurred, then the plasma membrane of MOLM-13 cell must have not been spared in the process.
Even though most of the anti-microbial peptides have been found not to affect normal cells, rather affect the tumor cells, Gomesin: which is a peptide containing a disulfide bridge which builds a stable amphipathic Beta sheet structure: exhibits an enormous cytotoxic activity against both normal and tumor cells. The mechanism of action of this particular peptide has not been fully comprehended yet (Paredes et al., 2013). Because of the release of LDH by the gomesin peptide, it is suggested that cell death results from pores opening in the cell membrane or because of a detergent like effect which leads to permeabilization of the membrane to eukaryotic cells and bacteria. It was evidenced in other studies that cell death due to the gomesin peptide is associated to endocytosis of the gomesin and accumulation of calcium in the mitochondria until it is destroyed, this happens before the permeabilization of the membrane (Lu et al., 2016). QUB-1518 in this study, evidently had cytotoxic effects on the leukemic MOLM 13 cells, but it is not possible to distinguish whether cytotoxic effects will also be observed in normal cells in an in vivo set up.
Almost all peptides that suppress cancer are exported from the nucleus by a nuclear exporter protein such as the chromosome region maintenance 1 (CRM1) among other six exporters. The CRM1 inhibition, for instance, causes the forced retention of the nucleus, upregulation and the activating of more than one tumor suppressor protein (Paredes et al., 2013). A study on gastric cancer by Sexton et al. (2019) demonstrated that by targeting the inhibition of nuclear export protein exportin (XPO1) there will be realignment of various non-coding RNAs that could affect the growth of gastric cancer cells. In relation to this, after the plasma membrane of MOLM-13 was disintegrated possibly by the QUB-1518 there might have been inhibition of nuclear exporting protein therefore leading to the activation of several tumor suppressing proteins.
In a family of antibacterial peptides from fungi, peptaibols has been shown to induce cell death by the infiltration of calcium, which results in the μ-calpain activation and enhances the Bax translocation to the mitochondria, this triggers autophagy and apoptosis, with very little to no evidence of permeabilization of the cell (Damian et al., 2019). This calls for further studies to be done in this field, putting into consideration the differences in structures portrayed by theses peptides plays a critical role in their modes of action (Stone et al., 2017).
Due to the high rate of induction portrayed in leucocytes that are neoplastic, it is possible that cancerous cells rely on the growth promoting signals or/and anti- apoptotic signaling pathways that are mediated by anti leukemic peptide nuclear export (Hoskin & Ramamoorthy, 2008). Apparently, normal cells do not share an equal level of pathway dependence, hence, causing a high level of resistance to inhibition of the peptide. Therefore, nuclear sequestration of the peptide that is forced and treated causes a shift in balance of the anti-survival signals therefore leading to cell death of the tumor, but this process is well tolerated by other normal cells of the body, this implies there is a difference in dependence on one, two or more pathways (Etchin et al., 2013). Which simply means that since normal cells have a different pathway from tumor cells, it is possible for the anti-leukemic peptide to attack tumor cells leaving out the normal cells.
The main limitation of the study is that the mechanism beneath the potential of peptide QUB 1518 is yet to be elucidated. There is not enough literature on antimicrobial peptides that have antileukemic effects therefore it is not possible to fully comprehend the mechanism of action of the QUB-1518. Another limitation is that it was not possible to determine whether QUB 1518 only induces anti leukemic activity on the malignant cells living out normal cells or whether it is cytotoxic against both normal and malignant cells
Future Studies:
To perform the anti-leukemic effects of the anti-microbial peptide QUB 1518 against both normal and malignant cells in an in vitro set up. This will be done to determine whether the peptide has an active potency selection against abnormal cells and leaves out the normal cells or is toxic to both normal and abnormal cells.

In addition to the antileukemic peptides, there are novel compounds that have been isolated from sources that are natural like the violacein and the antimicrobial peptides which are undergoing investigation. These compounds have furthermore offered possibility for the innovation of novel classes of therapeutic agents for treatment of cancer, inclusive of leukemias, because of their immunomodulatory and cytotoxic abilities, hence, opening fresh avenues for the treatment of cancer.
It has been demonstrated in various studies on Leukemia that acute leukemia generates immunosuppressive environment that bypass mechanisms of immunosurveillance. Immunotherapy, therefore, has a higher potential as a more competent and an alternative that is less toxic to present anti leukemic treatment. The study utilized vaccination with a vaccine containing leukemic cells that are whole and exposed to RT53, which activates immunogenic death of the cell, that was cushioned against in vivo leukemia development, both therapeutically and prophylactically. In future, it will be a milestone to develop a vaccine from treated leukemic cells that prevents the development of leukemic cells in the body once vaccination is done.
CONCLUSION
Generally, there is a trend in the treatment of individuals with acute myeloid leukemia that is gradually shifting from the conventional chemotherapeutical drugs toward the use of antimicrobial peptides to target malignant cells. The emergence of novel drugs, the strategies of treatment and the circumvention of mechanisms of resistance are very important targets of continuous clinical investigations. Development of resistance to several drugs is not uncommon with leukemia cases, mostly due to expression of specific glycoproteins which are responsible for shipping antileukemic drugs such as: Etoposide and anthracyclines; out of the plasma membrane causing high levels of genes like MDRI to be linked with lowered levels of chemotherapeutic drugs in the tumor cells.
Recent research on cancer has supported the use of peptides with anti-cancerous activity since most of the chemotherapeutical drugs had adverse side effects on patients, such as bleeding easily, diarrhea, fatigue, sore on the mouth, hair loss, fever and dry mouth amongst others. Even though the peptide trials might have a predisposition to anorexia, this could be mitigated by intake of enough food supplements and can be reversed once the dosing stops. This is better than the toxicity experienced with chemotherapeutic drugs on normal cells which are hematopoietic in nature. Also, the inefficiency of most chemotherapeutic drugs can be attributed to various factors as decreased drug uptake, apoptosis resistance, metabolism of the drug, presence of cancer cells and increased efflux. This necessitates the development of more non-conventional approached to the treatment of leukemia.
QUB 1518 has been shown to have antileukemic effects against the AML (MOLM-13) cells. It is therefore wise to consider the peptide as a drug candidate for the treatment of acute myeloid leukemia.
REFERENCES
- Habault, J., Kaci, A., Pasquereau-Kotula, E., Fraser, C., Chomienne, C., Dombret, H., ... & Poyet, J. L. (2020). Prophylactic and therapeutic antileukemic effects induced by the AAC-11-derived Peptide RT53. OncoImmunology, 9(1), 1728871.
- McGowan, E. M., Alling, N., Jackson, E. A., Yagoub, D., Haass, N. K., Allen, J. D., & Martinello-Wilks, R. (2011). Evaluation of cell cycle arrest in estrogen responsive MCF-7 breast cancer cells: pitfalls of the MTS assay. PloS one, 6(6), e20623.
- Döhner, H., Weisdorf, D. J., & Bloomfield, C. D. (2015). Acute myeloid leukemia. New England Journal of Medicine, 373(12), 1136-1152.
- Ley, T. J., Ding, L., Walter, M. J., McLellan, M. D., Lamprecht, T., Larson, D. E., ... & Harris, C. C. (2010). DNMT3A mutations in acute myeloid leukemia. New England Journal of Medicine, 363(25), 2424-2433.
- Appelbaum, F. R., Gundacker, H., Head, D. R., Slovak, M. L., Willman, C. L., Godwin, J. E., ... & Petersdorf, S. H. (2006). Age and acute myeloid leukemia. Blood, 107(9), 3481-3485.
- Stone, R. M., Mandrekar, S. J., Sanford, B. L., Laumann, K., Geyer, S., Bloomfield, C. D., ... & Lo-Coco, F. (2017). Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. New England Journal of Medicine, 377(5), 454-464.
- Etchin, J., Sanda, T., Mansour, M. R., Kentsis, A., Montero, J., Le, B. T., ... & Shacham, S. (2013). KPT‐330 inhibitor of CRM 1 (XPO 1) ‐mediated nuclear export has selective anti‐leukaemic activity in preclinical models of T‐cell acute lymphoblastic leukaemia and acute myeloid leukaemia. British journal of haematology, 161(1), 117-127.
- Hoskin, D. W., & Ramamoorthy, A. (2008). Studies on anticancer activities of antimicrobial peptides. Biochimica et biophysica acta, 1778(2), 357–375.
- Yan, J., Liang, X., Bai, C., Zhou, L., Li, J., Wang, K., ... & Zhao, L. (2018). NK-18, a promising antimicrobial peptide: anti-multidrug resistant leukemia cells and LPS neutralizing properties. Biochimie, 147, 143-152.
- Paredes-Gamero, E. J., Nogueira-Pedro, A., Miranda, A., & Justo, G. Z. (2013). Hematopoietic modulators as potential agents for the treatment of leukemia. Front Biosci (Elite Ed), 5, 130-140.
- Lu, Y., Zhang, T. F., Shi, Y., Zhou, H. W., Chen, Q., Wei, B. Y., ... & Fu, C. Y. (2016). PFR peptide, one of the antimicrobial peptides identified from the derivatives of lactoferrin, induces necrosis in leukemia cells. Scientific reports, 6, 20823.
- Arab-Bafrani, Z., Shahbazi-Gahrouei, D., Abbasian, M., & Fesharaki, M. (2016). Multiple MTS Assay as the Alternative Method to Determine Survival Fraction of the Irradiated HT-29 Colon Cancer Cells. Journal of medical signals and sensors, 6(2), 112–116.
- Buri, M. V., Torquato, H. F. V., Barros, C. C., Ide, J. S., Miranda, A., & Paredes‐Gamero, E. J. (2017). Comparison of Cytotoxic Activity in Leukemic Lineages Reveals Important Features of β‐Hairpin Antimicrobial Peptides. Journal of cellular biochemistry, 118(7), 1764-1773.
- O'Connor, S., Szwej, E., Nikodinovic-Runic, J., O'Connor, A., Byrne, A. T., Devocelle, M., ... & Zinn, M. (2013). The anti-cancer activity of a cationic anti-microbial peptide derived from monomers of polyhydroxyalkanoate. Biomaterials, 34(11), 2710-2718.
- Snauwaert, S., Vandekerckhove, B., & Kerre, T. (2013). Can immunotherapy specifically target acute myeloid leukemic stem cells? Oncoimmunology, 2(2), e22943.
- Trivedi, R., Müller, G. A., Rathore, M. S., Mishra, D. P., & Dihazi, H. (2016). Anti-leukemic activity of shikonin: role of ERP57 in shikonin induced apoptosis in acute myeloid leukemia. Cellular Physiology and Biochemistry, 39(2), 604-616.
- Johnson-Arbor, K., Patel, H., & Dubey, R. (2019). Doxorubicin. In StatPearls [Internet]. StatPearls Publishing.
- Xie, W., Noble, J. A., & Zisserman, A. (2018). Microscopy cell counting and detection with fully convolutional regression networks. Computer methods in biomechanics and biomedical engineering: Imaging & Visualization, 6(3), 283-292.
- LeGresley, M., & McDermott, G. (2010). Counting chamber methods for quantitative phytoplankton analysis-haemocytometer, Palmer-Maloney cell and Sedgewick-Rafter cell. UNESCO (IOC Manuals and Guides), 25-30.
- Sexton, R., Mahdi, Z., Chaudhury, R., Beydoun, R., Aboukameel, A., Khan, H. Y., Baloglu, E., Senapedis, W., Landesman, Y., Tesfaye, A., Kim, S., Philip, P. A., & Azmi, A. S. (2019). Targeting Nuclear Exporter Protein XPO1/CRM1 in Gastric Cancer. International journal of molecular sciences, 20(19), 4826.
- Marie, D., Simon, N., & Vaulot, D. (2005). Phytoplankton cell counting by flow cytometry. Algal culturing techniques, 1, 253-267.
- Farhane, Z., Bonnier, F., & Byrne, H. J. (2017). Monitoring doxorubicin cellular uptake and trafficking using in vitro Raman microspectroscopy: short and long-time exposure effects on lung cancer cell lines. Analytical and bioanalytical chemistry, 409(5), 1333-1346.
- Zhang, H., Han, D., Lv, T., Liu, K., Yang, Y., Xu, X., & Chen, Y. (2019). Novel peptide myristoly-CM4 induces selective cytotoxicity in leukemia K562/MDR and Jurkat cells by necrosis and/or apoptosis pathway. Drug design, development and therapy, 13, 2153–2167.
- Riss TL, Moravec RA, Niles AL, et al. Cell Viability Assays. (2013) [Updated 2016 Jul 1]. In: Sittampalam GS, Grossman A, Brimacombe K, et al., editors. Assay Guidance Manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004
- Damiani, E., Solorio, J. A., Doyle, A. P., & Wallace, H. M. (2019). How reliable are in vitro IC50 values? Values vary with cytotoxicity assays in human glioblastoma cells. Toxicology letters, 302, 28-34.
Take a deeper dive into Quality Assurance in Health Care Management with our additional resources.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



