Necroptosis: Viral Defense Mechanism
Introduction
1.1. Definition of Necrosis, Pyroptosis, Necroptosis and Autophagy
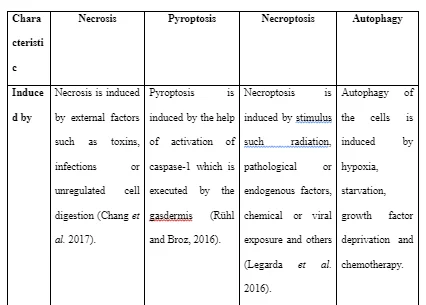
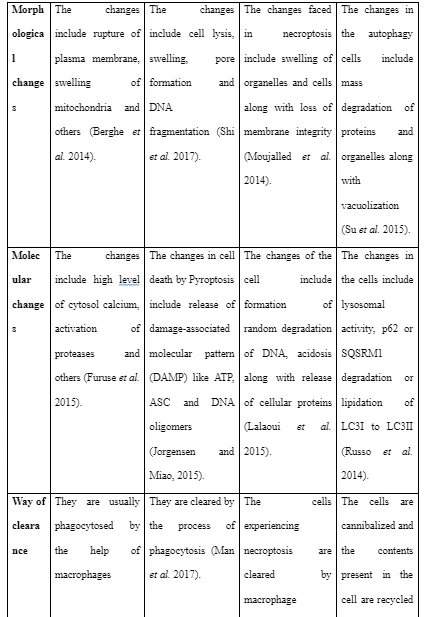
Necrosis is referred to as the premature death of cells through autolysis due to injury or response to lack of blood supply, infections, chemicals and others (Banoth and Sutterwala, 2017). Pyroptosis is referred as a highly inflammatory form of cell death in a programmed way which occurs frequently when infection happens with intracellular pathogens and is likely to be going to take part in the antimicrobial response (Doitsh et al. 2014). Necroptosis is referred to the viral defence mechanism which helps the cell to execute suicide in presence of an inhibitor of viral caspase in a caspase-independent way (Pasparakis and Vandenabeele, 2015). Autophagy is referred as the natural physiological process of the body which involves with cell destruction in the body that is done to maintain homeostasis or normal protein degradation and turnover of new information from the destroyed cellular organelles (An et al. 2014).

1.2. Apoptosis
Apoptosis is the way of programmed cell death which happens in the multi-cellular organisms. As mentioned by Gali-Muhtasib et al. (2015), cells which undergo apoptosis are seen to get initially shrinked and develop bubble-like projections on the cell surface. The DNA present in the nucleus of the apoptotic cell is fragmented into smaller pieces along with fragmentation of the cellular organelles. The cell at the end then splits into smaller chunks in the form of cellular debris which are engulfed by the macrophages. As argued by Peng et al. (2015), apoptosis leads to clearance of damaged or cancerous or infected cells. This helps in protecting the body from infections and disease. Apoptosis is also seen to play a key part in the maintenance and development of an effective immune system. This is because when T and B cells are produced they are put to test if they act against their body’s own defence system. The cells which act against the defence system are instantly eliminated out through the help of apoptosis (Mariño et al. 2014). Moreover, during detection of a pathogen in the body huge number of pathogen-specific immune cells is released into the body for the purpose of destruction of the pathogeṇ. However, after the pathogen is removed the pathogen-specific immune cells are removed by apoptosis to help the body maintain homeostasiss.
1.2.1. Intrinsic pathway
The intrinsic pathway is usually initiated during radiotherapy or chemotherapy. Thus, it is activated when a range of endogenous as well as exogenous stimuli like DNA damage, oxidative stress, ischemia are experienced by the body. They play a key role in the elimination of damaged cells (Joo et al. 2015). The intrinsic pathway in apoptosis is induced by direct activation of caspase-3 or through cleavage of BH3 interacting domain death agonist which results in dysfunction of the mitochondria and subsequent release of cytochrome-C accompanied by activation of caspase-3 and 9. The delivery of stress signals in the intrinsic pathway includes activation of proapoptoticc Bcl-2 family proteins which subsequently by interact and later inactivate anti-apoptotic Bcl-2 proteins (Yee et al. 2014). The proapoptoticc proteins include Bcl-2 associated X protein (Bax), Bcl-2 family apoptosis regulator (Bok) and Bcl-2homologouss antagonistic killer (Bak) whereas the anti-apoptotic proteins include Bcl-2, Bcl-XL and Mcl-1. The pro-apoptotic molecules later cause permeabilizationn of outer part of the mitochondrial membrane which leads to efflux cytochrome-C and bound to Apaf-1 along with initiator caspase-9 in the cytosol for the formation of apoptosome complex. In this process, the Bcl-2 and Bcl-XL performs the task to inhibit release of cytochrome-C whereas the Bak, Bid and Bax are seen to be involved in promoting its release from the mitochondria. The complex formed is seen to hydrolyse adenosine triphosphatee for cleavage and activation of caspase-9. The initiator caspase-9 is then seen to active and cleaves executioner caspase-3/6/7 that result the cell to undergo apoptosis (Park et al. 2016).
1.2.2. Extrinsic pathway
The extrinsic pathway is seen to be stimulated by external death-induced signals for apoptosis. Each of the cells is seen to possess receptors in the plasma membrane which are specific for each stimulus and therefore the extrinsic pathway is also called Receptor-Mediated programmed cell pathway. In most cases, Tumour Necrosis Factor (TNF) is the cytokine that is responsible for the external stimuli in initiating extrinsic pathway of apoptosis. The TNF is mostly provoked in the body when the body is exposed to toxic substances and elevated temperature (Ashkenazi, 2015). The extrinsic pathway of apoptosis which is mediated by TNF factor initially involves binding of the TNF to the TNF receptor-1 (TNFR-1) on the plasma membrane of the cell. The TNFR-1 belongs to a member of the death receptor family which activates apoptotic signal process. The TNFR-1 is a transmembrane receptor which has external domain for bonding of ligand along with has a cytosol domain and it is presented in the form of a pre-assembler trimer (Nair et al. 2014). The cytosolic domain present in each subunit of TNFR-1 contains 70 amino acid segment known as death domain (Chattopadhyay et al. 2014). The binding of the Tumour Necrosis factor (TNF) to the TNFR-1 causes a change in the death domain which results to recruit many apoptotic-related adaptor protein factors. In order to further activate the death domain, the cytosolic adaptor TRADD and FADD along with procaspase-8 residue is bound in forming the multi-protein complex. The homologous region of TNFR-1, TRADD and FADD are seen to interact with one another. The FADD and caspase-8 also have homologous region which interacts in generating an activated caspase-8. This activated caspase-8 acts as an initiator in activation of the extrinsic pathway of apoptosis which activates caspase-3 which is the key apoptotic executioner creating self-destruction of cell (Richardson et al. 2017). The Fas-ligand is also seen to cause extrinsic apoptosis by activatingprocaspasee-8 which in turn activates the downstream executioner caspase (caspase-3) for inducing apoptosis (Ahmed et al. 2015).
1.2.3. NF-κB pathway role in apoptosis
Nuclear Factor κB (NF-κB) is a form of transcription factor related to a large genetic group that are involved in different pathway. In executing apoptosis, the NF-κB activates their inhibitor κB (I-κB) along with forms group with pro-apoptotic and anti-apoptotic genes. Later, the activated NF-κB results in transcription of the gene which encodes the inhibitor for apoptosis protein (IAP). The activation of the protein results in down-regulation of the activity of the cascade of caspase that form the core part of apoptosis (Dondelinger et al. 2015). The canonical NF-κB pathway which plays their role in apoptosis is induced by cytokine tumor necrosis factor α (TNF-α). The binding of TNF-α to the tumor necrosis factorreceptorr-1 (TNFR-1) which is a death receptor results in forming an initial complex that activates NF- κB. Later, a second complex is formed that activates caspase-8 which results in activation of the apoptotic process. The same TNF-α stimulation triggers two parallel pathway which is contrary in nature that is pro-apoptotic caspase cascade pathway along with anti-apoptotic NF- κB -I-κB-IAP pathway. Both of the pathway decides the cell survival or initiation of programmed cell death (Sun, 2017).

1.2.4 Caspases in relation to apoptosis
Caspasess belong to enzymes of the protease family who have essential role in executing programmed cell death and inflammation. The caspases found in mammals are divided into three groups that include initiator caspase (caspases 8, 2, 9 and 10), executioner caspase (caspases 7, 3 and 6) and inflammatory caspase (caspases 5,4,11, 12 and 1). The initiator caspasesacts to initiate the apoptotic process whereas the executioner caspases execute the proteolysis which leads to apoptosis. The capases are initially synthesized in the form of inactive pro-caspases which is later cleaved as well as activated toresponde to death receptors, granzyme B and apoptosome stimuli. The activated caspases are then going to cleave various substrates that include nuclear protein, downstream caspases, plasma membrane protein and others which are going to let it ultimately execute cell death (Rongvaux et al. 2014).
1.2.5 Inhibitors of apoptosis (IAP)
The inhibitors of apoptosis (IAP) proteins are the structural and functional family of proteins which executes their function as an endogenous inhibitor of apoptosis or programmed cell death. All the IAPs have a common feature that is presence of BIR in one to three of their copies (Ebert et al. 2015). The IAPs that are usually found in humans include XIAP, NAIP, Livin, C-OAP2, Survivin and others.
Bcl-2 family of proteins
The Bcl-2 family of proteins can either promote or inhibit programmed cell death and the members of this group of proteins are characterised by BL-2 homologous domain. The members of the family who are responsible for inhibiting apoptosis are Bcl-xl, Bcl-w and Bcl-2 itself. Bcl-2 is one of the well-knownn inhibitor apoptosis found in oncogenes. The Bcl-2 is seen to inhibit apoptosis either by disruption of the channels which allows leaving of pro-apoptotic factor from the mitochondria or by controlling the cleavage and activation of the caspases (Czabotar et al. 2014).
Survivin
Survivinn or Baculoviral inhibitor apoptosis repeat-containing 5 (BIRC5) is protein of the IAP family which inhibit the activation of caspases resulting in negative regulation of programmed cell death or apoptosis (Han et al. 2015).

1.3. Discuss bacteria that induce and inhibit apoptosis for multiplication, survival and dissemination in different immune cell types
There are different ways in which different bacteria inhibit or induce apoptosis for their survival, multiplication as well as dissemination in different types of immune cells. The Mycobacterium tuberculosis uses either of two key pathways to protect the cell against apoptosis. The pathways include either induction of TLR-2-dependent activation that results in NF-κB pathway for cell survival or enhanced production of soluble TNF receptor 2 (sTNFR2) that acts to neutralise the pro-apoptotic activity of TNF-α (Srinivasan et al. 2014). Moreover, components released from the mycobacterial wall of M.tuberculosis are also seen to influence apoptosis and the influence is usually seen to be caused by lipoarabinomannan – LAM. As a result of influence from the molecule, the apoptoticc cycle is activated thoroughly through phosphorylation of Bad which prevent the binding with the anti-apoptotic proteins Bcl-xl and Bcl-2 (Halder et al. 2015). The Legionella pneumophila is mainly seen to induce apoptosis through cell contact which might be done by binding of the pathogen with commonly found receptor on macrophages through cell-cell interaction or by translocation of effector protein by using Dot/Icm secretion system. In L.pneumophila, more than 330 effector proteins are encoded which is used by the pathogen to interfere with fusion of the pathogen’s vacuole with the endosomes of the host in turn protecting the pathogen against cell lysis (Kawamoto et al. 2017). The Neisseriaa meningitidis is seen to release membrane protein porin PorB to prevent the release of cytochrome-C to inhibit apoptosis. The cytochrome-C is one of the key components that act to initiate apoptosis (Deo et al. 2018). The Salmonella releases effector SopB through typeIII secretion system which results in activating phosphatidylinositol 3-kinase/Akt (PI3K/Akt) pathway. The pathway reacts to prevent the release of cytochrome-C resulting in inhibition of apoptosis (Robinson and Aw, 2016). The Anaplasma is also seen to activate PI3/Akt pathway along with activation of the nuclear factor kappa B (NF-κB). This NF-κB result in preventing the release of cytochrome-C along with activates the IAPs to inhibit apoptosis (Ayllón et al. 2015).
References
- Ahmed, W., Philip, P.S., Attoub, S. and Khan, G., 2015. Epstein–Barr virus-infected cells release Fas ligand in exosomal fractions and induce apoptosis in recipient cells via the extrinsic pathway. Journal of General Virology, 96(12), pp.3646-3659.
- An, Z., Tassa, A., Thomas, C., Zhong, R., Xiao, G., Fotedar, R., Tu, B.P., Klionsky, D.J. and Levine, B., 2014. Autophagy is required for G1/G0 quiescence in response to nitrogen starvation in Saccharomyces cerevisiae. Autophagy, 10(10), pp.1702-1711.
- Ayllón, N., Villar, M., Galindo, R.C., Kocan, K.M., Šíma, R., López, J.A., Vazquez, J., Alberdi, P., Cabezas-Cruz, A., Kopáček, P. and de la Fuente, J., 2015. Systems biology of tissue-specific response to Anaplasma phagocytophilum reveals differentiated apoptosis in the tick vector Ixodes scapularis. PLoS genetics, 11(3), p.e1005120.
- Berghe, T.V., Linkermann, A., Jouan-Lanhouet, S., Walczak, H. and Vandenabeele, P., 2014. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nature reviews Molecular cell biology, 15(2), p.135.
- Chang, X., Wang, L., Wang, Z., Wu, S., Zhu, X., Hu, S., Wang, Y., Yu, J. and Chen, G., 2017. TRADD mediates the tumor necrosis factor-induced apoptosis of L929 cells in the absence of RIP3. Scientific reports, 7(1), p.16111.
- Czabotar, P.E., Lessene, G., Strasser, A. and Adams, J.M., 2014. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nature reviews Molecular cell biology, 15(1), p.49.
- Doitsh, G., Galloway, N.L., Geng, X., Yang, Z., Monroe, K.M., Zepeda, O., Hunt, P.W., Hatano, H., Sowinski, S., Muñoz-Arias, I. and Greene, W.C., 2014. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature, 505(7484), p.509.
- Ebert, G., Allison, C., Preston, S., Cooney, J., Toe, J.G., Stutz, M.D., Ojaimi, S., Baschuk, N., Nachbur, U., Torresi, J. and Silke, J., 2015. Eliminating hepatitis B by antagonizing cellular inhibitors of apoptosis. Proceedings of the National Academy of Sciences, 112(18), pp.5803-5808.
- Furuse, M., Nonoguchi, N., Kawabata, S., Miyatake, S.I. and Kuroiwa, T., 2015. Delayed brain radiation necrosis: pathological review and new molecular targets for treatment. Medical molecular morphology, 48(4), pp.183-190.
- Halder, P., Kumar, R., Jana, K., Chakraborty, S., Ghosh, Z., Kundu, M. and Basu, J., 2015. Gene expression profiling of Mycobacterium tuberculosis Lipoarabinomannan‐treated macrophages: A role of the Bcl‐2 family member A1 in inhibition of apoptosis in mycobacteria‐infected macrophages. IUBMB life, 67(9), pp.726-736.
- Joo, J.H., Ueda, E., Bortner, C.D., Yang, X.P., Liao, G. and Jetten, A.M., 2015. Farnesol activates the intrinsic pathway of apoptosis and the ATF4-ATF3-CHOP cascade of ER stress in human T lymphoblastic leukemia Molt4 cells. Biochemical pharmacology, 97(3), pp.256-268.
- Kawamoto, Y., Morinaga, Y., Kimura, Y., Kaku, N., Kosai, K., Uno, N., Hasegawa, H. and Yanagihara, K., 2017. TNF-α inhibits the growth of Legionella pneumophila in airway epithelial cells by inducing apoptosis. Journal of Infection and Chemotherapy, 23(1), pp.51-55.
- Legarda, D., Justus, S.J., Ang, R.L., Rikhi, N., Li, W., Moran, T.M., Zhang, J., Mizoguchi, E., Zelic, M., Kelliher, M.A. and Blander, J.M., 2016. CYLD proteolysis protects macrophages from TNF-mediated auto-necroptosis induced by LPS and licensed by type I IFN. Cell reports, 15(11), pp.2449-2461.
- Park, Y.J., Choi, C.I., Chung, K.H. and Kim, K.H., 2016. Pharbilignan C induces apoptosis through a mitochondria-mediated intrinsic pathway in human breast cancer cells. Bioorganic & medicinal chemistry letters, 26(19), pp.4645-4649.
- Richardson, J.S.M., Aminudin, N. and Malek, S.N.A., 2017. Chalepin: A compound from Ruta angustifolia L. pers exhibits cell cycle arrest at S phase, suppresses nuclear factor-kappa B (NF-κB) pathway, signal transducer and activation of transcription 3 (STAT3) phosphorylation and extrinsic apoptotic pathway in non-small cell lung cancer carcinoma (A549). Pharmacognosy magazine, 13(3), p.489.
- Russo, R., Cassiano, M.G.V., Ciociaro, A., Adornetto, A., Varano, G.P., Chiappini, C., Berliocchi, L., Tassorelli, C., Bagetta, G. and Corasaniti, M.T., 2014. Role of D-limonene in autophagy induced by bergamot essential oil in SH-SY5Y neuroblastoma cells. PloS one, 9(11), p.e113682.
- Strzyz, P., 2016. Cell Death: Molecular insights into execution of necroptosis. Nature Reviews Molecular Cell Biology, 17(3), p.134.
- Yee, C., Yang, W. and Hekimi, S., 2014. The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell, 157(4), pp.897-909.
- Zhuang, H., Tian, W., Li, W., Zhang, X., Wang, J., Yang, Y., Liu, X., Xia, Z., Feng, D. and Zhang, L., 2016. Autophagic Cell Death and Apoptosis Jointly Mediate Cisatracurium Besylate-Induced Cell Injury. International journal of molecular sciences, 17(4), p.515.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



